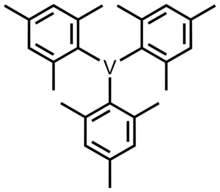
Trimesitylvanadium (mesityl or Mes = 2,4,6-trimethylphenyl) is one of the organovanadium complexes with vanadium in an oxidation state of 3. This compound was first synthesized by W. Seidel and G. Kreisel in 1974.[1][2] To prepare this compound, VCl3(THF)3 (THF = tetrahydrofuran) was reacted with Grignard reagent MesMgBr to form a blue solution at room temperature.[3][4] It is precipitated by the addition of dioxane, which results in a blue solid. It is thermally stable, but it is also an air-sensitive compound.

| |
| |
| Names | |
|---|---|
| IUPAC name
1,3,5-trimethylbenzene-6-ide;vanadium
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C27H33V | |
| Molar mass | 408.503 g·mol−1 |
| Appearance | blue solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Structure
editThe fact that trimesitylvanadium is recrystallized with THF adduct is due to the strong interaction between vanadium and oxygen. The bond length of the V-O bond is 2.069 Å.[4] According to Pyykkö's atomic radii periodic trend, 1.97 Å would be expected for a single bond between vanadium and oxygen.[5] This suggests that this V-O bond is not fully of single bond, but it is still close enough that it is considered a strong interaction, resulting in the formation of an adduct in recrystallization. However, THF can be easily dissociated during the reaction. Experiments found that THF in trimesitylvanadium was exchanged with either pyridine or 2,2'-bipyridine when the product was exposed to either chemical.[2] A crystal structure revealed VMes3(THF) with trigonal pyramidal or pseudo-tetrahedral geometry.[6][7]
Insertion reaction by using reactive V-C bond
editThe V-C σ bond in trimesitylvanadium is so reactive that it undergoes insertion reaction of several molecules. Rozzoli et al. investigated the reactivity of V(Mes)3THF with CO, CO2, and tBuCN.[3] When V(Mes)3THF is reacted with CO, it undergoes reductive elimination and forms MesC(=O)Mes as a product. Excess of CO will also result in the formation of V(CO)6 as a side product. For CO2 and pivalonitrile (or tBuCN) as a reagent, they are inserted between the V-C bond. Since V(Mes)3THF is air- and water-sensitive, when the product from the insertion of tBuCN is exposed to water and/or O2, it undergoes reductive elimination to form imine and amine. These reactions reveal examples of small molecule activation reactions.
Deoxygenation with vanadium
editVanadium (III) is known to be oxophilic transition metal.[8][9][10] In vanadium(III) species, V(Mes)3(THF) undergoes deoxygenation of styrene oxide.[11] The styrene oxide turns into styrene while vanadium(III) species becomes vanadyl(V) species (O=V(Mes)3). This product with V(Mes)3(THF) can form μ-oxo complex in toluene as a solution. This unique compound has a magnetic moment of 1.65 μB per vanadium at 288 K and a V-O-V stretch vibration of 680 cm−1.[11] However, this μ-oxo complex is decomposed under polar coordinating solvent such as pyridine (= py), in which it forms tetramesitylvanadium [V(Mes)4] and pyridine-coordinated complex [(Mes)2V(py)2] with C2 symmetry. For [(Mes)2V(py)2], the bond length of the V-C bond is much longer than trimesitylvanadium and trimesitylvanadyl complexes. The μ-oxo complex, tetramesitylvanadium, and pyridine-coordinated complex are examples of vanadium(IV) complexes.
Deoxygenation by trimesitylvanadium can also be done for coordinated nitric oxide. In (ON)Cr(N-i-Pr2)3 (i-Pr = isopropyl), introducing V(Mes)3THF in toluene leads to cleavage of N=O bond to form CrΞO complex and μ-oxo vanadium complex.[6][12] This reaction reduces and cleaves the NO bond by using five electrons.
Binding dinitrogen by trimesitylvanadium
editThis study was motivated after finding vanadium-containing nitrogenase, which needed a better understanding of the activation of dinitrogen.[6][13] Floriani et al. attempted the reduction of dinitrogen by using V(Mes)3(THF). After reducing with Na metal in diglyme, Na[V(Mes)3] is reacted with N2 to form N2-bound species V(Mes)3N2Na. This product with Na[V(Mes)3] eventually formed N2-bridge product [Na(diglyme)2][Na(p-Mes)2(p-N2)V2(Mes)2]. Na ion is located in between aromatic π-conjugation in the mesityl group. Crystallographic analysis revealed that N-N in the product is longer (1.280(21) Å) than free N2 (1.0968 Å). Moreover, this product has a magnetic moment of 1.69 μB per vanadium atom at 293 K.[13] This is due to the reduction of vanadium upon bonding with dinitrogen in a bridging fashion. This reaction was also observed with K metal, resulting in the product with a magnetic moment of 1.83 μB per vanadium atom at 293 K.
Application
editTrimesitylvanadium is a precursor for organometallic fragments in hexagonally packed mesoporous silica (HMS) as a hydrogen storage source.[14] This vanadium-loaded HMS can absorb 2.68 H2 per vanadium center before the hydrogenation effect and 2.74 H2 per vanadium center after hydrogenation.
References
edit- ^ Seidel, Wolfgang; Kreisel, Günter (January 1974). "Zur Darstellung von Trimesitylvanadin". Zeitschrift für Chemie. 14 (1): 25. doi:10.1002/zfch.19740140116. ISSN 0044-2402.
- ^ a b Seidel, W.; Kreisel, G. (November 1977). "Arylvanadin(III)-Verbindungen, III. Darstellung und Eigenschaften von Triarylvanadin-Verbindungen". Zeitschrift für anorganische und allgemeine Chemie. 435 (1): 146–152. doi:10.1002/zaac.19774350119. ISSN 0044-2313.
- ^ a b c d e Vivanco, Marilin; Ruiz, Javier; Floriani, Carlo; Chiesi-Villa, Angiola; Rizzoli, Corrado (May 1993). "Chemistry of the vanadium-carbon .sigma. bond. 1. Insertion of carbon monoxide, isocyanides, carbon dioxide, and heterocumulenes into the V-C bond of tris(mesityl)vanadium(III)". Organometallics. 12 (5): 1794–1801. doi:10.1021/om00029a041. ISSN 0276-7333.
- ^ a b Gambarotta, Sandro; Floriani, Carlo; Chiesi-Villa, Angiola; Guastini, Carlo (1984). "A tri-σ-aryl vanadium(<scp>III</scp>) derivative: structural determination of trimesitylvanadium(<scp>III</scp>)–tetrahydrofuran". J. Chem. Soc., Chem. Commun. (13): 886–887. doi:10.1039/c39840000886. ISSN 0022-4936.
- ^ Pyykkö, Pekka; Atsumi, Michiko (January 2009). "Molecular Single-Bond Covalent Radii for Elements 1–118". Chemistry – A European Journal. 15 (1): 186–197. doi:10.1002/chem.200800987. ISSN 0947-6539. PMID 19058281.
- ^ a b c d e Cummins, Christopher C. (January 1997), Karlin, Kenneth D. (ed.), Three-Coordinate Complexes of "Hard" Ligands: Advances in Synthesis, Structure and Reactivity, Progress in Inorganic Chemistry, vol. 47 (1 ed.), Wiley, pp. 685–836, doi:10.1002/9780470166482.ch7, ISBN 978-0-471-24039-6, retrieved 2024-02-25
- ^ Koschmieder, Stefan U.; Wilkinson, Geoffrey (1991-01-01). "Homoleptic and related aryls of transition metals". Polyhedron. 10 (2): 135–173. doi:10.1016/S0277-5387(00)81585-5. ISSN 0277-5387.
- ^ Griffin, Samuel E.; Schafer, Laurel L. (2020-04-20). "Vanadium Pyridonate Catalysts: Isolation of Intermediates in the Reductive Coupling of Alcohols". Inorganic Chemistry. 59 (8): 5256–5260. doi:10.1021/acs.inorgchem.0c00071. ISSN 0020-1669. PMID 32223129.
- ^ Groysman, Stanislav; Goldberg, Israel; Goldschmidt, Zeev; Kol, Moshe (2005-07-01). "Vanadium(III) and Vanadium(V) Amine Tris(Phenolate) Complexes". Inorganic Chemistry. 44 (14): 5073–5080. doi:10.1021/ic050084i. ISSN 0020-1669. PMID 15998036.
- ^ King, R. Bruce; Schaefer, H. F.; Liu, Zhaohui; Li, Qian-Shu; Xie, Yaoming (2008-04-15). "The oxophilicity of vanadium in unsaturated homoleptic binuclear vanadium carbonyl structures". Journal of Organometallic Chemistry. F.A. Cotton Memorial Issue. 693 (8): 1502–1509. doi:10.1016/j.jorganchem.2007.10.058. ISSN 0022-328X.
- ^ a b c d Vivanco, Marilin; Ruiz, Javier; Floriani, Carlo; Chiesi-Villa, Angiola; Rizzoli, Corrado (May 1993). "Chemistry of the vanadium-carbon .sigma. bond. 2. Oxovanadium(IV) and oxovanadium(V) containing metal-to-carbon .sigma. bonds". Organometallics. 12 (5): 1802–1810. doi:10.1021/om00029a042. ISSN 0276-7333.
- ^ a b Odom, Aaron L.; Cummins, Christopher C.; Protasiewicz, John D. (June 1995). "Nitric Oxide Cleavage: Synthesis of Terminal Chromium(VI) Nitrido Complexes via Nitrosyl Deoxygenation". Journal of the American Chemical Society. 117 (24): 6613–6614. doi:10.1021/ja00129a034. ISSN 0002-7863.
- ^ a b c Ferguson, Richard; Solari, Euro; Floriani, Ccrlo; Chiesi-Villa, Angiola; Rizzoli, Corrado (March 1993). "Fixierung und Reduktion von N 2 durch V II und V III : Synthese und Struktur von Mesityl(distickstoff)vanadium-Komplexen". Angewandte Chemie. 105 (3): 453–455. Bibcode:1993AngCh.105..453F. doi:10.1002/ange.19931050331. ISSN 0044-8249.
- ^ Hamaed, Ahmad; Mai, Hung Van; Hoang, Tuan K. A.; Trudeau, Michel; Antonelli, David (2010-05-13). "Functionalized Porous Silicas with Unsaturated Early Transition Metal Moieties as Hydrogen Storage Materials: Comparison of Metal and Oxidation State". The Journal of Physical Chemistry C. 114 (18): 8651–8660. doi:10.1021/jp1013048. ISSN 1932-7447.