Tuinal was the brand name of a discontinued combination drug composed of two barbiturate sodium salts (secobarbital and amobarbital) in equal proportions.
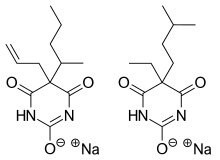 | |
| Combination of | |
|---|---|
| Secobarbital | Short-acting barbiturate |
| Amobarbital | Intermediate-acting barbiturate |
| Clinical data | |
| Trade names | Tuinal |
| Routes of administration | By mouth |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| 3D model (JSmol) | |
| |
| |
Tuinal was introduced as a sedative-hypnotic (sleeping pill) medication in the late 1940s by Eli Lilly. It was also used in obstetrics for childbirth.[1][2] It was produced in brightly colored half-reddish orange and half-turquoise blue gelatin capsule form (bullet-shaped Pulvules) for oral administration. Individual capsules contained 50 mg, 100 mg, or 200 mg of barbiturate salts. The combination of a short-acting barbiturate, secobarbital, with an intermediate-acting barbiturate, amobarbital, aimed to provide "a rapid yet prolonged hypnotic action".[3]
Eli Lilly has discontinued the manufacture of Tuinal in the United States due to the diminishing use of barbiturates (largely replaced by the benzodiazepine family of drugs) in outpatient treatment, and its widespread abuse.[4] Currently, Valleant Labs markets secobarbital capsules only. Flynn Pharma of Ireland no longer manufactures Tuinal, Seconal (secobarbital), or Amytal (amobarbital). Amytal has been discontinued, though injectable forms of amobarbital sodium remains.
Abuse
editTuinal saw widespread abuse as a recreational drug from the 1960s through the 1980s. The pill was known colloquially under the street names "tuies", "tumies", "double trouble", "blue tips", " F-66's" (which were the markings on Lilly's capsule), "rainbows", "beans", "nawls" and "jeebs".[5] It came in the form of bullet-shaped capsules, half-reddish orange and half-turquoise blue. Like other barbiturate depressants, Tuinal promotes physical and psychological dependency beginning after one week of regular use and carries a high risk of overdose.[6] It was reported in the 1980s as one of the most common ways of self-poisoning.[7] Abuse of this particular drug tapered off after it was discontinued by manufacturers in the late 1990s.
Tuinal is classified as a Schedule II drug under the Controlled Substances Act in the United States, meaning it requires a prescription from a licensed practitioner.
References
edit- ^ Waters AB (1947). "Pethidine In Labour". The British Medical Journal. 2 (4514): 71–72. doi:10.1136/bmj.2.4514.71-b. ISSN 0007-1447. JSTOR 20370143. PMC 2055200. PMID 20344014.
- ^ "Front Matter". The British Medical Journal. 1 (4539). 1948. ISSN 0007-1447. JSTOR 25361874.
- ^ "Front Matter". The American Journal of Nursing. 47 (5): 1–24. 1947. ISSN 0002-936X. JSTOR 3457169.
- ^ Mitchell M, Willingham EJ, Atkins WA (2012). Key K (ed.). The Gale Encyclopedia of Mental Health. Vol. 1 (3rd ed.). Detroit, MI: Gale eBooks. p. 171. ISBN 9781414490144. Retrieved November 4, 2022.
- ^ Bigelow BC, ed. (2006). UXL Encyclopedia of Drugs and Addictive Substances. Detroit, MI: Gale eBooks. p. 99. ISBN 9781414404448. Retrieved November 4, 2022.
- ^ Evans JI, Lewis SA, Gibb IA, Cheetham M (November 1968). "Sleep and birbiturates: some experiments and observations". British Medical Journal. 4 (5626): 291–293. doi:10.1136/bmj.4.5626.291. PMC 1912258. PMID 4301261.
- ^ Ray JE, Reilly DK, Day RO (April 1986). "Drugs involved in self-poisoning: verification by toxicological analysis". The Medical Journal of Australia. 144 (9): 455–457. doi:10.5694/j.1326-5377.1986.tb101047.x. PMID 2871482. S2CID 24568454.