| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Nitric acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| 1576 | |||
| KEGG | |||
| MeSH | Nitric+acid | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2031 | ||
| |||
| |||
| Properties | |||
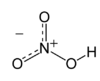
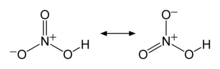
| HNO3 | |||
| Molar mass | 63.012 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Density | 1.5129 g cm-3 | ||
| Melting point | −42 °C (−44 °F; 231 K) | ||
| Boiling point | 83 °C (181 °F; 356 K) | ||
| Completely miscible | |||
| Acidity (pKa) | -1.4 | ||
Refractive index (nD)
|
1.397 (16.5 °C) | ||
| 2.17 ± 0.02 D | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions
|
Nitrous acid | ||
Other cations
|
Sodium nitrate Potassium nitrate Ammonium nitrate | ||
| Supplementary data page | |||
| User:Beetstra/test (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Tracking categories (test):
blah