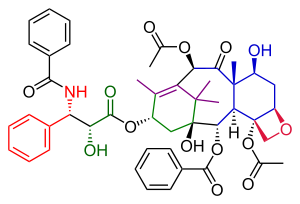
The Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996[1] two years after the first two efforts described in the Holton Taxol total synthesis and the Nicolaou Taxol total synthesis. Combined they provide a good insight in the application of organic chemistry in total synthesis.
Danishefsky's route to Taxol has many similarities with that of Nicolaou. Both are examples of convergent synthesis with a coupling of the A and the C ring from two precursors. The main characteristic of the Danishefsky variant is the completion of the oxetane D ring onto the cyclohexanol C ring prior to the construction of the 8-membered B ring. The most prominent starting material is the Wieland-Miescher ketone. This compound is commercially available as a single enantiomer and the single chiral group present in this molecule is able to drive the entire sequence of organic reactions to a single optically active Taxol endproduct. The final step, the tail addition is identical to that of Nicolaou and is based on Ojima chemistry.[2]
In terms of raw material shopping, this taxol molecule consists of the aforementioned Wieland-Miescher ketone, 2-methyl-3-pentanone, lithium aluminium hydride, osmium tetroxide, phenyllithium, pyridinium chlorochromate, the Corey-Chaykovsky reagent and acryloyl chloride. Key chemical transformations are the Johnson-Corey-Chaykovsky reaction and the Heck reaction.
D Ring synthesis
editScheme 1 shows the synthesis of the oxetane D ring from the C ring starting from the Wieland-Miescher ketone 1. Organic reduction of this ketone with sodium borohydride provides the alcohol 2 which is protected as an acyl group in 3 with acetic anhydride, DMAP and pyridine. The ketone group is also protected as the acetal 4 with glycol catalyzed by naphthalenesulfonic acid. The acetyl group is replaced by a TBS group by first deprotection with sodium ethoxide in ethanol and reprotection with tert-butyldimethylsilyltriflate and lutidine accompanied by an alkene isomerization. The double bond in 4 is activated to a hydroxyl group in 5 by a hydroboration reaction followed by oxidation with hydrogen peroxide. The hydroxyl group is then oxidized to ketone 6 by action of pyridinium dichromate. With all the sensitive functional groups disabled, the methylene group required for the oxetane ring D can now be provided by the Corey-Chaykovsky reagent which converts the carbonyl group to the oxirane 7. Aluminium isopropoxide opens the epoxide ring and forms the allyl alcohol 8 after elimination of water. Two more hydroxyl groups are generated by oxidation of the newly formed double bond with osmium tetroxide and N-Methylmorpholine N-oxide as re-oxidant. This reaction lacks stereospecifity and the yield of triol 9 with the correct stereochemistry is therefore reduced. The primary alcohol is then converted into the silyl ether 10 with trimethylsilyl chloride in pyridine and the secondary alcohol is modified to the triflate 11 with triflic anhydride. A good nucleophile and a good leaving group are now in place in the correct anti-conformation for the final oxetane ring formation step to 12 in ethylene glycol at reflux temperature.
|
|
| Scheme 1 |
|---|
C Ring synthesis
editIn the next phase starting from the WM ketone the C ring is modified by a ring-opening procedure from which two anchoring points are formed for fusion with the A ring. In Scheme 2 the alcohol 12 is protected by a benzyl group with benzyl bromide, sodium hydride and a quaternary ammonium salt as phase transfer catalyst. In 13 the acetal protecting group is removed from the ketone with p-toluenesulfonic acid. This ketone (14) forms the silyl enol ether 15 by reaction with trimethylsilyltriflate and a Rubottom oxidation introduces an acyloin group in 16. Ring opening by oxidative cleavage with lead tetraacetate in methanol generates a methyl ester group and an aldehyde group in 17. In the next step the aldehyde is protected as an acetal with methanol and collidine p-toluenesulfonate (CPTS) and the ester is reduced to the primary alcohol 18 with Lithium aluminium hydride. The hydroxyl group is converted in a Grieco elimination to the selenide in 19 which on oxidation with hydrogen peroxide gives the alkene 20. Ozonolysis with ozone and triphenylphosphine provides the aldehyde 21.
|
|
| Scheme 2 |
|---|
AB Ring synthesis
editThe A ring is a cyclohexane ring with two functional groups, a vinyl lithium group and a masked enolate for hooking up with the C ring forming the 8 membered B ring similar to the Nicolaou effort. Starting materials for this synthesis in Scheme 3 are ethyl isopropyl ketone 22 which forms enamine 23 with morpholine. This enamine reacts with acryloyl chloride in a combined nucleophilic conjugate addition and nucleophilic acyl substitution to the cyclohexene 24. In the next step the morpholine group is removed by hydrolysis to the dione 25. Reaction with hydrazine in triethylamine and ethanol affords the hydrazone 26 and reaction with iodine and DBN gives the iodide 27 in a hydrazone iodination. This reaction step is complicated because not the mono-ene is isolated but the diene 28 in an unexpected dehydrogenation. The ketone group is converted into the cyanohydrin 29 with trimethylsilyl cyanide, potassium cyanide and a crown ether and in the last step iodine is replaced by lithium in the vinyl lithium 30 by reaction with tert-butyllithium in THF at −78 °C.
|
|
| Scheme 3 |
|---|
As shown in Scheme 4, the bottom part of the taxol B ring synthesis is a nucleophilic addition of the vinyl lithium 30 group of ring A with the ring C aldehyde group of 21. In 30 the ketone group is deprotected by action of TBAF which removes the trimethylsilyl group in 31. In the next step the double bond is oxidized with MCPBA to the epoxide 32 . This epoxide is then hydrogenated with hydrogen over palladium on carbon to the diol 33 which is protected in the next step as the cyclic carbonate ester 34 by reaction with carbonyl diimidazole and sodium hydride in dimethylformamide. These two alcohol groups are part of the final taxol molecule.
The alkene reduction of 34 to 35 with L-Selectride corrects the unexpected outcome of the A ring hydrazone iodinization. The ketone is converted into the vinyl triflate 36 when reacted with phenyl triflimide and potassium hexamethyldisilazide in THF at −78 °C. This is one of the functional groups taking part in the Heck reaction. For the generation of the other reactive group the acetal group is deprotected with pyridinium tosylate to the carbonyl group in 37 which is subsequently converted to the terminal alkene 38 in a Wittig reaction with methylenetriphenylphosphorane. The intramolecular Heck reaction of 38 to 39 with Tetrakis(triphenylphosphine)palladium(0) and potassium carbonate in acetonitrile at reflux completes the second ring closing reaction for the B ring.
|
|
| Scheme 4 |
|---|
B Ring elaboration
editThe second part of the B ring synthesis (Scheme 5) was concerned with correct chemistry for the newly formed ethylene bridge connecting the A and C rings. After Scheme 4, this bridge had an exocyclic methylene group but in the ultimate taxol molecule this bridge is an α-acyl ketone. The required conversion was accomplished in the following 10 steps.
The TBS protecting group in 39 was not compatible with future functional groups and was replaced by a TES (triethylsilyl) group in 40 through the intermediate hydroxyl group. Next the A ring double bond was converted into oxirane 41 with meta-Chloroperoxybenzoic acid The oxirane also served as a protecting group in preparation for modifications of the exocyclic alkene. In the next two steps the benzyl protecting group in oxirane 41 had served its purpose and was replaced by an acyl group (acetic anhydride,4-Dimethylaminopyridine and pyridine) in carbonate ester 43 through alcohol 42 (hydrogenation over palladium on carbon). Carbonate ester 43 was opened by reaction with phenyllithium to form α-hydroxybenzoate ester 44. The cleavage of the exocyclic double bond was accomplished by formation of osmate ester 45 with osmium tetraoxide and pyridine and subsequent oxidative cleavage with lead tetraacetate formed ketone 46. The epoxide protecting group was subsequently removed with samarium (II) iodide and acetic anhydride in tetrahydrofuran at −78 °C to form ketone 47. The reaction of ketone 47 with potassium tert-butoxide formed the enolate and subsequent reaction with phenylseleninic anhydride produced hydroxy ketone 48. This oxidation step is comparable to allylic oxidation with selenium dioxide. In the final step the hydroxyl group was acylated to produce α-acyl ketone 49.
|
|
| Scheme 5 |
|---|
Tail addition
editThe tail addition step in this synthesis (Scheme 6) was identical to that in the Nicolaou tail addition and based on Oijma chemistry. The A ring was functionalized with a hydroxyl group through PCC oxidation of α-acyl ketone 49 to ketone 50 and subsequent reduction to alcohol 51 with sodium borohydride. Reaction of alcohol 51 with Ojima lactam 52 and a concluding silyl deprotection step at two TES positions in triethylsilyl-protected paclitaxel 53 produced Taxol. Because the correct stereochemistry was already introduced in the WM-ketone this synthetic Taxol had the same optical rotation as the natural compound.
|
|
| Scheme 6 |
|---|
See also
editReferences
edit- ^ Danishefsky, S. J.; Masters, J. J.; Young, W. B.; Link, J. T.; Snyder, L. B.; Magee, T. V.; Jung, D. K.; Isaacs, R. C. A.; Bornmann, W. G.; Alaimo, C. A.; Coburn, C. A.; Di Grandi, M. J. "Total synthesis of baccatin III and taxol." J. Am. Chem. Soc. 1996, 118, 2843–2859. doi:10.1021/ja952692a
- ^ Ojima, I.; Habus, I.; Zhao, M.; Zucco, M.; Park, Y. H.; Sun, C. M.; Brigaud, T. "New and efficient approaches to the semisynthesis of taxol and its C-13 side chain analogs by means of β-lactam synthon method." Tetrahedron 1992, 48, 6985–7012. doi:10.1016/S0040-4020(01)91210-4