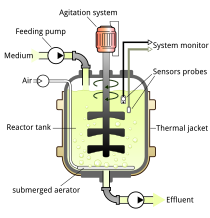
A bioreactor is a vessel or system in which a chemical process is carried out which involves cultured cells or biochemically active substances derived from organisms. These process may either be aerobic, in which oxygen is continuously fed into and dispersed within the reactor, or anaerobic, without the addition of oxygen. Bioreactors are commonly cylindrical, ranging in size from liters at the lab scale to cubic meters at the industrial scale.[1] In many industries, bioreactor refers to a device or system designed to culture cells that have been genetically modified in order produce a desired biological product such as biosynthetic insulin, or monoclonal antibodies. Bioreactor type devices are frequently used in industries such as tissue engineering, biochemical/bioprocess engineering, sewage treatment and biopharmaceuticals.
On the basis of mode of operation, a bioreactor may be classified as batch in which both inputs and products are periodically removed, fed batch in which only inputs are continuously added or continuous, in which both inputs and products are continuously cleared from the system.
Bioreactor Designs
editContinuos Stirred Tank
editA stirred tank bioreactors (STR) is the most conventional type of bioreactor employed in a variety of industries. Fundamentally, these consist of a cylindrical vessel with a central motor driven impeller. Stirred tank reactors rely on the mechanical agitation provided by a central set of impellers to maintain the homogeneity of the environment inside of the reactor. In the case of an aerobic process, oxygen is added to the system at the bottom of the column through a sparker. The air bubbles are broken up and dispersed the impeller to provide uniform oxygenation.[1][2]
Bubble Column
editIn a similar fashion to stirred tank bioreactors, bubble column reactors introduce oxygen or an alternative gas at the bottom end of the vessel through perforated pipes or plates or a sparger. In contrast to stirred tank reactors, bubble columns don't employ any kind of mechanical agitation. The flow rate of the gas alone influences performance factors and mixes the contents of the vessel.[2] Bubble columns (BCs) typically required less floor space and less maintenance compared to traditional stirred tank reactors. An additional modification that is typically included is the widening of the upper end of the vessel to encourage gas separation.[3]
Packed Bed
editPacked bed bioreactors consist of a bed of solid particles that make up the media in which biological reactions take place. Biocatalysts are typically placed onto the surface of the particles or within the packed matrix. The particles used may vary great but can typically separated into the categories of porous or non-porous as well as rigid or compressible. A nutrient rich fluid is made to flow over the packed particles in order to foster interaction with the catalyst as well as remove any product material generated. Fluid flow may be upward or downward within the vessel, however downward flow is sometimes favored due to the assistance of gravity. The concentration of nutrients within the bed can be controlled by regulating the fluid flow. Packed bed reactors are preferred in cases where accumulation of the product inhibits the ongoing reaction since the product accumulation within the vessel remains minimal.[1][2]
Photobioreactor
editA photobioreactor (PBR) is a bioreactor which incorporates some type of light source (that may be natural sunlight or artificial illumination). Virtually any translucent container could be called a PBR, however the term is most commonly used to define a closed system, as opposed to an open storage tank or pond. Photobioreactors are used to grow small phototrophic organisms such as cyanobacteria, algae, or moss plants. These organisms use light through photosynthesis as their energy source and do not require sugars or lipids as an energy source. Consequently, risk of contamination with other organisms like bacteria or fungi is lower in photobioreactors when compared to bioreactors for heterotroph organisms. Typical designs of photobioreactors may incorporate an array of transparent tubes or panels known as 'solar receivers'. The cell culture is then circulated through the solar receivers through the use of a pumping mechanism. This ensures adequate access to sunlight and prevents the formation of dense sediments.[2]
Uses in Industry
editSewage Treatment
editConventional sewage treatment utilizes bioreactors to undertake the main purification processes. In some of these systems, a chemically inert medium with very high surface area is provided as a substrate for the growth of biological film. Separation of excess biological film takes place in settling tanks or cyclones. In other systems aerators supply oxygen to the sewage and biota to create activated sludge in which the biological component is freely mixed in the liquor in "flocs". In these processes, the liquid's Biochemical Oxygen Demand (BOD) is reduced sufficiently to render the contaminated water fit for reuse. The biosolids can be collected for further processing, or dried and used as fertilizer. An extremely simple version of a sewage bioreactor is a septic tank whereby the sewage is left in situ, with or without additional media to house bacteria. In this instance, the waste itself is the primary host for the bacteria.[4]
Algae Biofuel Production
editIn algae biofuel production, enclosed photobioreactors offer an alternative to 'open ponds' for the growth of the microalgae culture which are later harvested for the creation of biodiesel. These have been employed to mitigate evaporation and contamination issues that may occur with open ponds. These reactors are made of transparent material, as most photobioreactors, and can be placed outdoors or in a greenhouse facility. Designs typically aim to maximize surface area to ensure adequate access to natural light.
The most widely used reactor for this purpose is a tubular design. Tubes are aligned with the direction of sunlight and are generally less than 10 cm in diameter to maximize light penetration. The culture medium is circulated through these tubes and then back to a collection reservoir. Here, a portion of the algae is harvested and a portion is circulated to maintain the culture.
Aside from mitigating evaporation and contamination from the environment, other advantages of closed a bioreactor over an open pond include the biomass productivity and ease of harvesting. The biomass production of a photobioreactor can be as much as 13 times greater than that of a traditional raceway pond. Since the algal biomass is typically about 30 times as concentrated, harvesting is made easier. The disadvantages of a bioreactor are a higher cost and typically lower volume when compared to an open pond.
Biopharmaceuticals
editBioreactors are a necessary component for the upstream bioprocess in the production of biopharmaceutical drugs, or those that are derived from living cells or other biochemical processes. The upstream bioprocess consists of the cultivation of genetically modified cells in a controlled environment. Within this industry, bioreactors may be involved in the production of vaccines, therapeutics, gene therapies, and cell therapies.[5]
Due to the highly sensitive nature of drug product quality, bioreactors used must be able to meet regulatory specifications and offer precise conditions controls. Economic considerations are also key in designs due to the high production costs and end prices of many biopharmaceutical treatments.
While traditional stainless steel bioreactors have long been used in the biopharmaceutical industry, recent trends have spurred a shift towards more single-use plastic designs which are cheap and disposable. This shift is the result of the introduction of higher concentration cell cultures and a shift towards more niche, lower volume drugs. Especially in the production of cell and gene therapies where drugs are custom formulated to individual patients, single-use bioreactor designs become more practical. These also allow for faster turn around during the production process.[6]
Tissue Engineering
editMany cells and tissues, especially mammalian ones, must have a surface or other structural support in order to grow, and agitated environments are often destructive to these cell types and tissues. More complex tissues also require highly specific conditions and biological signals to foster their development.This poses a challenge when the goal is to culture larger quantities of cells for therapeutic production purposes, and a significantly different design is needed compared to industrial bioreactors used for growing protein expression systems such as yeast and bacteria.[7]
Many research groups have developed novel bioreactors for growing specialized tissues and cells on a structural scaffold, in attempt to recreate organ-like tissue structures in-vitro, or in a laboratory setting. Among these are included tissue bioreactors that can grow heart tissue, skeletal muscle tissue, ligaments, cancer tissue models, and others. Currently, scaling production of these specialized bioreactors for industrial use remains challenging and is an active area of research.[7]
For more information on artificial tissue culture, see tissue engineering.
Design Considerations
editBioreactor design is a relatively complex engineering task, which is studied in the discipline of biochemical/bioprocess engineering. Under optimum conditions, the microorganisms or cells are able to perform their desired function with limited production of impurities. The environmental conditions inside the bioreactor, such as temperature, nutrient concentrations, pH, and dissolved gases (especially oxygen for aerobic fermentations) affect the growth and productivity of the organisms. The temperature of the fermentation medium is maintained by a cooling jacket, coils, or both. Particularly exothermic fermentations may require the use of external heat exchangers. Nutrients may be continuously added to the fermenter, as in a fed-batch system, or may be charged into the reactor at the beginning of fermentation. The pH of the medium is measured and adjusted with small amounts of acid or base, depending upon the fermentation. For aerobic (and some anaerobic) fermentations, reactant gases (especially oxygen) must be added to the fermentation. Since oxygen is relatively insoluble in water (the basis of nearly all fermentation media), air (or purified oxygen) must be added continuously. The action of the rising bubbles helps mix the fermentation medium and also "strips" out waste gases, such as carbon dioxide. In practice, bioreactors are often pressurized; this increases the solubility of oxygen in water. In an aerobic process, optimal oxygen transfer is sometimes the rate limiting step. Oxygen is poorly soluble in water—even less in warm fermentation broths—and is relatively scarce in air (20.95%). Oxygen transfer is usually helped by agitation, which is also needed to mix nutrients and to keep the fermentation homogeneous. Gas dispersing agitators are used to break up air bubbles and circulate them throughout the vessel.[8]
Fouling can harm the overall efficiency of the bioreactor, especially the heat exchangers. To avoid it, the bioreactor must be easily cleaned. Interior surfaces are typically made of stainless steel for easy cleaning and sanitation. Typically bioreactors are cleaned between batches, or are designed to reduce fouling as much as possible when operated continuously. Heat transfer is an important part of bioreactor design; small vessels can be cooled with a cooling jacket, but larger vessels may require coils or an external heat exchanger.[9]
Components
editFermentation Vessel
editThe fermentation vessel of a bioreactor is the large cylindrical chamber that contains the operational fluid. Typically enclosed at both the top and bottom, the vessel is intended to maintain specific operating conditions. Vessels are typically made of glass or stainless steel due to their non-toxic and corrosion proof properties. Glass vessels are more common in smaller scale lab settings. Stainless steel vessels are more typical for large scale industry operations.[1]
Impeller
editThe impeller of a bioreactor is the rotating component present in stirred tank bioreactors. Impellers are typically made up of a set of impeller blades driven by a centralized motor. The impeller serves to provide a uniform concentration of the components present in the reactor media (ie. a uniform suspension of cells in their growth media) through continuous stirring. Impellers also interact with the aeration system of the reactor, serving to break up and evenly distribute air bubbles.[1]
Types of impellers that may be used in stirred tank reactors include:
- Turbine Disc
- Variable Pitch
Cooling Jacket
editThe exterior of the bioreactor vessel is typically fitted with a cooling jacket or insulated outer layer that allows for temperature regulation of the system. Cooled or heated water can be run through the jacket to regulate temperature. In cases of larger reactors, internal coils filled with cooled water or steam allow for more even temperature control.[10]
Baffles
editBaffles are metal strips attached radially to the inner wall of the fermentation vessel. These are intended to stop the formation of a vortex within the reactor fluid and improve overall aeration.[10]
Controlling Devices
editA wide array of sensors along with input and output regulation devices control specific parameters of operation. Controlled parameters will vary widely based on application but may include: temperature, oxygen concentration, pH, cell mass, key nutrient concentration and output product concentration.[10]
Sparger
editA sparger is used to introduce sterile air into the fermentation vessel of a stirred tank bioreactor. The sparger mechanism contains small holes (typically 5-10 mm in diameter) through which pressurized air enters the vessel.[1]
Types of spargers that may be used include:
- Porous sparger
- Nozzle sparger
- Combined sparger-agitator
Modeling
editMathematical models act as an important tool in various bio-reactor applications including wastewater treatment. These models are useful for planning efficient process control strategies and predicting the future plant performance. Moreover, these models are beneficial in education and research areas.[11]
History
editChaim Weizmann, a Russian-born biochemist is believed to have developed the first 'fermentor' for the production of acetone during World War I. Considered to be the father of industrial fermentation, Weizmann has previously developed the acetone-butanol-ethanol fermentation process, which allows all three compounds to be produced through the process of bacterial fermentation. His method for the production of acetone was important to the production of cordite explosive propellants for the British during World War I.[10]
In 1944, De Beeze and Liebmann used the first large scale bioreactor (above 20 liter capacity) for the production of yeast.[10]
In 1949, the New BrunswickTool and Dye Company build an improved version of a 'test-tube shaking apparatus' being used by graduate students of Dr. Selman Waksman at Rutgers University. They called their new design the "New Brunswick Shaker".[12]
References
edit- ^ a b c d e f Magar, Sanjogta Thapa (2021-09-07). "Bioreactor- Definition, Design, Principle, Parts, Types, Applications, Limitations". Microbe Notes. Retrieved 2021-11-24.
- ^ a b c d "Bioreactors Types: 6 Types of Bioreactors used in Bioprocess Technology". Biology Discussion. 2015-09-21. Retrieved 2021-11-23.
- ^ "Bubble Column Bioreactors", An Introduction to Bioreactor Hydrodynamics and Gas-Liquid Mass Transfer, John Wiley & Sons, Ltd, pp. 124–167, 2014, doi:10.1002/9781118869703.ch7, ISBN 978-1-118-86970-3, retrieved 2021-11-23
- ^ Narayanan, C. M.; Narayan, Vikas (2019-12-11). "Biological wastewater treatment and bioreactor design: a review". Sustainable Environment Research. 29 (1): 33. doi:10.1186/s42834-019-0036-1. ISSN 2468-2039.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "What Is a Bioreactor? - Biotech Blog | Pall Corporation". Pall. Retrieved 2021-11-23.
- ^ Macdonald, Gareth John (2019-07-01). "Bioreactor Design Adapts to Biopharma's Changing Needs". GEN - Genetic Engineering and Biotechnology News. Retrieved 2021-11-24.
- ^ a b "Bioreactors". ebersmedical.com. Retrieved 2021-11-26.
- ^ "Bioreactor Design and Bioprocess Controls for Industrialized Cell Processing". BioProcess International. 2012-06-01. Retrieved 2021-11-26.
- ^ "Fouling in Heat Exchangers: Learn Causes, Detection, and Prevention". Central States Industrial. Retrieved 2021-11-26.
- ^ a b c d e "Fermentor (Bioreactor): History, Design and Its Construction". Biology Discussion. 2016-09-16. Retrieved 2021-11-26.
- ^ "Modeling Bioreactor Performance - Bioprocess Development Forum". www.processdevelopmentforum.com. Retrieved 2021-11-26.
- ^ "BIOREACTORS IN BIOTECHNOLOGY - History". bioreactors.weebly.com. Retrieved 2021-11-26.
