Pharmacokinetics
editThe oral bioavailability of amphetamine varies with gastrointestinal pH;[1] it is well absorbed from the gut, and bioavailability is typically 90%.[2] Amphetamine is a weak base with a pKa of 9.9;[3] consequently, when the pH is basic, more of the drug is in its lipid soluble free base form, and more is absorbed through the lipid-rich cell membranes of the gut epithelium.[3][1] Conversely, an acidic pH means the drug is predominantly in a water-soluble cationic (salt) form, and less is absorbed.[3] Approximately 20% of amphetamine circulating in the bloodstream is bound to plasma proteins.[4] Following absorption, amphetamine readily distributes into most tissues in the body, with high concentrations occurring in cerebrospinal fluid and brain tissue.[5]
The half-lives of amphetamine enantiomers differ and vary with urine pH.[3] At normal urine pH, the half-lives of dextroamphetamine and levoamphetamine are 9–11 hours and 11–14 hours, respectively.[3] Highly acidic urine will reduce the enantiomer half-lives to 7 hours;[5] highly alkaline urine will increase the half-lives up to 34 hours.[5] The immediate-release and extended release variants of salts of both isomers reach peak plasma concentrations at 3 hours and 7 hours post-dose respectively.[3] Amphetamine is eliminated via the kidneys, with 30–40% of the drug being excreted unchanged at normal urinary pH.[3] When the urinary pH is basic, amphetamine is in its free base form, so less is excreted.[3] When urine pH is abnormal, the urinary recovery of amphetamine may range from a low of 1% to a high of 75%, depending mostly upon whether urine is too basic or acidic, respectively.[3] Following oral administration, amphetamine appears in urine within 3 hours.[5] Roughly 90% of ingested amphetamine is eliminated 3 days after the last oral dose.[5]
Lisdexamfetamine is a prodrug of dextroamphetamine.[6][7] It is not as sensitive to pH as amphetamine when being absorbed in the gastrointestinal tract.[7] Following absorption into the blood stream, lisdexamfetamine is completely converted by red blood cells to dextroamphetamine and the amino acid L-lysine by hydrolysis via undetermined aminopeptidase enzymes.[7][6][8] This is the rate-limiting step in the bioactivation of lisdexamfetamine.[6] The elimination half-life of lisdexamfetamine is generally less than 1 hour.[7][6] Due to the necessary conversion of lisdexamfetamine into dextroamphetamine, levels of dextroamphetamine with lisdexamfetamine peak about one hour later than with an equivalent dose of immediate-release dextroamphetamine.[6][8] Presumably due to its rate-limited activation by red blood cells, intravenous administration of lisdexamfetamine shows greatly delayed time to peak and reduced peak levels compared to intravenous administration of an equivalent dose of dextroamphetamine.[6] The pharmacokinetics of lisdexamfetamine are similar regardless of whether it is administered orally, intranasally, or intravenously.[6][8] Hence, in contrast to dextroamphetamine, parenteral use does not enhance the subjective effects of lisdexamfetamine.[6][8] Because of its behavior as a prodrug and its pharmacokinetic differences, lisdexamfetamine has a longer duration of therapeutic effect than immediate-release dextroamphetamine and shows reduced misuse potential.[6][8]
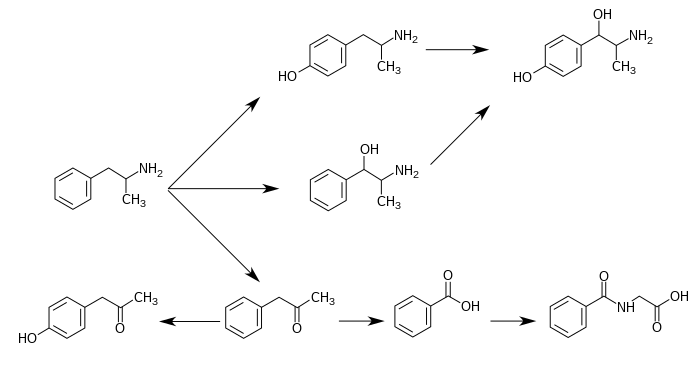
CYP2D6, dopamine β-hydroxylase (DBH), flavin-containing monooxygenase 3 (FMO3), butyrate-CoA ligase (XM-ligase), and glycine N-acyltransferase (GLYAT) are the enzymes known to metabolize amphetamine or its metabolites in humans.[9] Amphetamine has a variety of excreted metabolic products, including 4-hydroxyamphetamine, 4-hydroxynorephedrine, 4-hydroxyphenylacetone, benzoic acid, hippuric acid, norephedrine, and phenylacetone.[3][10] Among these metabolites, the active sympathomimetics are 4-hydroxyamphetamine,[11] 4-hydroxynorephedrine,[12] and norephedrine.[13] The main metabolic pathways involve aromatic para-hydroxylation, aliphatic alpha- and beta-hydroxylation, N-oxidation, N-dealkylation, and deamination.[3][14] The known metabolic pathways, detectable metabolites, and metabolizing enzymes in humans include the following:
Metabolic pathways of amphetamine in humans[sources 1]
|
Note
edit- ^ 4-Hydroxyamphetamine has been shown to be metabolized into 4-hydroxynorephedrine by dopamine beta-hydroxylase (DBH) in vitro and it is presumed to be metabolized similarly in vivo.[16][21] Evidence from studies that measured the effect of serum DBH concentrations on 4-hydroxyamphetamine metabolism in humans suggests that a different enzyme may mediate the conversion of 4-hydroxyamphetamine to 4-hydroxynorephedrine;[21][23] however, other evidence from animal studies suggests that this reaction is catalyzed by DBH in synaptic vesicles within noradrenergic neurons in the brain.[24][25]
sources
editRefs
edit- ^ a b "Adderall XR- dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine sulfate and amphetamine aspartate capsule, extended release". DailyMed. Shire US Inc. 17 July 2019. Retrieved 22 December 2019.
- ^ Patel VB, Preedy VR, eds. (2022). Handbook of Substance Misuse and Addictions. Cham: Springer International Publishing. p. 2006. doi:10.1007/978-3-030-92392-1. ISBN 978-3-030-92391-4.
Amphetamine is usually consumed via inhalation or orally, either in the form of a racemic mixture (levoamphetamine and dextroamphetamine) or dextroamphetamine alone (Childress et al. 2019). In general, all amphetamines have high bioavailability when consumed orally, and in the specific case of amphetamine, 90% of the consumed dose is absorbed in the gastrointestinal tract, with no significant differences in the rate and extent of absorption between the two enantiomers (Carvalho et al. 2012; Childress et al. 2019). The onset of action occurs approximately 30 to 45 minutes after consumption, depending on the ingested dose and on the degree of purity or on the concomitant consumption of certain foods (European Monitoring Centre for Drugs and Drug Addiction 2021a; Steingard et al. 2019). It is described that those substances that promote acidification of the gastrointestinal tract cause a decrease in amphetamine absorption, while gastrointestinal alkalinization may be related to an increase in the compound's absorption (Markowitz and Patrick 2017).
- ^ a b c d e f g h i j k l m Cite error: The named reference
Amphetamine FDA Pharmacokineticswas invoked but never defined (see the help page). - ^ Wishart, David S.; Djombou Feunang, Yannick; Guo, An Chi; Lo, Elvis J.; Marcu, Ana; Grant, Jason R.; Sajed, Tanvir; Johnson, Daniel; Li, Carin; Sayeeda, Zinat; Assempour, Nazanin; Iynkkaran, Ithayavani; Liu, Yifeng; Maciejewski, Adam; Gale, Nicola; Wilson, Alex; Chin, Lucy; Cummings, Ryan; Le, Diana; Pon, Allison; Knox, Craig; Wilson, Michael. "Amphetamine | DrugBank Online". DrugBank. 5.0.
- ^ a b c d e "Metabolism/Pharmacokinetics". Amphetamine. Hazardous Substances Data Bank. United States National Library of Medicine – Toxicology Data Network. Archived from the original on 2 October 2017. Retrieved 2 October 2017.
Duration of effect varies depending on agent and urine pH. Excretion is enhanced in more acidic urine. Half-life is 7 to 34 hours and is, in part, dependent on urine pH (half-life is longer with alkaline urine). ... Amphetamines are distributed into most body tissues with high concentrations occurring in the brain and CSF. Amphetamine appears in the urine within about 3 hours following oral administration. ... Three days after a dose of (+ or -)-amphetamine, human subjects had excreted 91% of the (14)C in the urine
- ^ a b c d e f g h i Ermer JC, Pennick M, Frick G (May 2016). "Lisdexamfetamine Dimesylate: Prodrug Delivery, Amphetamine Exposure and Duration of Efficacy". Clinical Drug Investigation. 36 (5): 341–356. doi:10.1007/s40261-015-0354-y. PMC 4823324. PMID 27021968.
- ^ a b c d "Vyvanse- lisdexamfetamine dimesylate capsule Vyvanse- lisdexamfetamine dimesylate tablet, chewable". DailyMed. Shire US Inc. 30 October 2019. Retrieved 22 December 2019.
- ^ a b c d e Dolder PC, Strajhar P, Vizeli P, Hammann F, Odermatt A, Liechti ME (2017). "Pharmacokinetics and Pharmacodynamics of Lisdexamfetamine Compared with D-Amphetamine in Healthy Subjects". Front Pharmacol. 8: 617. doi:10.3389/fphar.2017.00617. PMC 5594082. PMID 28936175.
Inactive lisdexamfetamine is completely (>98%) converted to its active metabolite D-amphetamine in the circulation (Pennick, 2010; Sharman and Pennick, 2014). When lisdexamfetamine is misused intranasally or intravenously, the pharmacokinetics are similar to oral use (Jasinski and Krishnan, 2009b; Ermer et al., 2011), and the subjective effects are not enhanced by parenteral administration in contrast to D-amphetamine (Lile et al., 2011) thus reducing the risk of parenteral misuse of lisdexamfetamine compared with D-amphetamine. Intravenous lisdexamfetamine use also produced significantly lower increases in "drug liking" and "stimulant effects" compared with D-amphetamine in intravenous substance users (Jasinski and Krishnan, 2009a).
- ^ Cite error: The named reference
Amphetamine amphetamine metabolismwas invoked but never defined (see the help page). - ^ a b Cite error: The named reference
Amphetamine Metaboliteswas invoked but never defined (see the help page). - ^ "Compound Summary". p-Hydroxyamphetamine. PubChem Compound Database. United States National Library of Medicine – National Center for Biotechnology Information. Retrieved 15 October 2013.
- ^ "Compound Summary". p-Hydroxynorephedrine. PubChem Compound Database. United States National Library of Medicine – National Center for Biotechnology Information. Retrieved 15 October 2013.
- ^ "Compound Summary". Phenylpropanolamine. PubChem Compound Database. United States National Library of Medicine – National Center for Biotechnology Information. Retrieved 15 October 2013.
- ^ "Pharmacology and Biochemistry". Amphetamine. Pubchem Compound Database. United States National Library of Medicine – National Center for Biotechnology Information. Retrieved 12 October 2013.
- ^ "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. Shire US Inc. December 2013. pp. 12–13. Retrieved 30 December 2013.
- ^ a b Glennon RA (2013). "Phenylisopropylamine stimulants: amphetamine-related agents". In Lemke TL, Williams DA, Roche VF, Zito W (eds.). Foye's principles of medicinal chemistry (7th ed.). Philadelphia, US: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 646–648. ISBN 9781609133450.
The simplest unsubstituted phenylisopropylamine, 1-phenyl-2-aminopropane, or amphetamine, serves as a common structural template for hallucinogens and psychostimulants. Amphetamine produces central stimulant, anorectic, and sympathomimetic actions, and it is the prototype member of this class (39). ... The phase 1 metabolism of amphetamine analogs is catalyzed by two systems: cytochrome P450 and flavin monooxygenase. ... Amphetamine can also undergo aromatic hydroxylation to p-hydroxyamphetamine. ... Subsequent oxidation at the benzylic position by DA β-hydroxylase affords p-hydroxynorephedrine. Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ^ Taylor KB (January 1974). "Dopamine-beta-hydroxylase. Stereochemical course of the reaction" (PDF). Journal of Biological Chemistry. 249 (2): 454–458. doi:10.1016/S0021-9258(19)43051-2. PMID 4809526. Retrieved 6 November 2014.
Dopamine-β-hydroxylase catalyzed the removal of the pro-R hydrogen atom and the production of 1-norephedrine, (2S,1R)-2-amino-1-hydroxyl-1-phenylpropane, from d-amphetamine.
- ^ Krueger SK, Williams DE (June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacology & Therapeutics. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602. PMID 15922018.
Table 5: N-containing drugs and xenobiotics oxygenated by FMO - ^ Cashman JR, Xiong YN, Xu L, Janowsky A (March 1999). "N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication". Journal of Pharmacology and Experimental Therapeutics. 288 (3): 1251–1260. PMID 10027866.
- ^ Santagati NA, Ferrara G, Marrazzo A, Ronsisvalle G (September 2002). "Simultaneous determination of amphetamine and one of its metabolites by HPLC with electrochemical detection". Journal of Pharmaceutical and Biomedical Analysis. 30 (2): 247–255. doi:10.1016/S0731-7085(02)00330-8. PMID 12191709.
- ^ a b c Sjoerdsma A, von Studnitz W (April 1963). "Dopamine-beta-oxidase activity in man, using hydroxyamphetamine as substrate". British Journal of Pharmacology and Chemotherapy. 20 (2): 278–284. doi:10.1111/j.1476-5381.1963.tb01467.x. PMC 1703637. PMID 13977820.
Hydroxyamphetamine was administered orally to five human subjects ... Since conversion of hydroxyamphetamine to hydroxynorephedrine occurs in vitro by the action of dopamine-β-oxidase, a simple method is suggested for measuring the activity of this enzyme and the effect of its inhibitors in man. ... The lack of effect of administration of neomycin to one patient indicates that the hydroxylation occurs in body tissues. ... a major portion of the β-hydroxylation of hydroxyamphetamine occurs in non-adrenal tissue. Unfortunately, at the present time one cannot be completely certain that the hydroxylation of hydroxyamphetamine in vivo is accomplished by the same enzyme which converts dopamine to noradrenaline.
- ^ Badenhorst CP, van der Sluis R, Erasmus E, van Dijk AA (September 2013). "Glycine conjugation: importance in metabolism, the role of glycine N-acyltransferase, and factors that influence interindividual variation". Expert Opinion on Drug Metabolism & Toxicology. 9 (9): 1139–1153. doi:10.1517/17425255.2013.796929. PMID 23650932. S2CID 23738007.
Figure 1. Glycine conjugation of benzoic acid. The glycine conjugation pathway consists of two steps. First benzoate is ligated to CoASH to form the high-energy benzoyl-CoA thioester. This reaction is catalyzed by the HXM-A and HXM-B medium-chain acid:CoA ligases and requires energy in the form of ATP. ... The benzoyl-CoA is then conjugated to glycine by GLYAT to form hippuric acid, releasing CoASH. In addition to the factors listed in the boxes, the levels of ATP, CoASH, and glycine may influence the overall rate of the glycine conjugation pathway.
- ^ Horwitz D, Alexander RW, Lovenberg W, Keiser HR (May 1973). "Human serum dopamine-β-hydroxylase. Relationship to hypertension and sympathetic activity". Circulation Research. 32 (5): 594–599. doi:10.1161/01.RES.32.5.594. PMID 4713201. S2CID 28641000.
The biologic significance of the different levels of serum DβH activity was studied in two ways. First, in vivo ability to β-hydroxylate the synthetic substrate hydroxyamphetamine was compared in two subjects with low serum DβH activity and two subjects with average activity. ... In one study, hydroxyamphetamine (Paredrine), a synthetic substrate for DβH, was administered to subjects with either low or average levels of serum DβH activity. The percent of the drug hydroxylated to hydroxynorephedrine was comparable in all subjects (6.5-9.62) (Table 3).
- ^ Freeman JJ, Sulser F (December 1974). "Formation of p-hydroxynorephedrine in brain following intraventricular administration of p-hydroxyamphetamine". Neuropharmacology. 13 (12): 1187–1190. doi:10.1016/0028-3908(74)90069-0. PMID 4457764.
In species where aromatic hydroxylation of amphetamine is the major metabolic pathway, p-hydroxyamphetamine (POH) and p-hydroxynorephedrine (PHN) may contribute to the pharmacological profile of the parent drug. ... The location of the p-hydroxylation and β-hydroxylation reactions is important in species where aromatic hydroxylation of amphetamine is the predominant pathway of metabolism. Following systemic administration of amphetamine to rats, POH has been found in urine and in plasma.
The observed lack of a significant accumulation of PHN in brain following the intraventricular administration of (+)-amphetamine and the formation of appreciable amounts of PHN from (+)-POH in brain tissue in vivo supports the view that the aromatic hydroxylation of amphetamine following its systemic administration occurs predominantly in the periphery, and that POH is then transported through the blood-brain barrier, taken up by noradrenergic neurones in brain where (+)-POH is converted in the storage vesicles by dopamine β-hydroxylase to PHN. - ^ Matsuda LA, Hanson GR, Gibb JW (December 1989). "Neurochemical effects of amphetamine metabolites on central dopaminergic and serotonergic systems". Journal of Pharmacology and Experimental Therapeutics. 251 (3): 901–908. PMID 2600821.
The metabolism of p-OHA to p-OHNor is well documented and dopamine-β hydroxylase present in noradrenergic neurons could easily convert p-OHA to p-OHNor after intraventricular administration.
- ^ Cite error: The named reference
Amphetamine Glycine conjugation reviewwas invoked but never defined (see the help page).