 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Daivonex, Dovonex, Sorilux, others |
| Other names | calcipotriene (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608018 |
| Pregnancy category |
|
| Routes of administration | Topical |
| Drug class | Form of vitamin D[1] |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 5 to 6% |
| Metabolism | Liver |
| Excretion | Biliary |
| Identifiers | |
| |
| Chemical and physical data | |
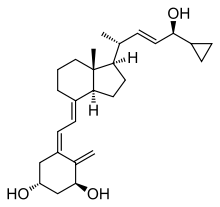
| Formula | C27H40O3 |
| Molar mass | 412.614 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Calcipotriol, also known as calcipotriene, is a medication used to treat psoriasis.[2] It is applied to the skin.[2] It is also available in combination with betamethasone.[2]
Common side effects include skin irritation, itchiness, and worsening psoriasis.[2] Other side effects may include increased sensitivity to UV radiation and high calcium.[2] Safety in pregnancy is unclear.[2] It is a manufactured derivative of calcitriol, a form of vitamin D.[1]
Calcipotriol was patented in 1985 and approved for medical use in 1991.[3] It is on the World Health Organization's List of Essential Medicines.[4] It is available as a generic medication.[5] In the United States 60 grams costs about 70 USD as of 2021.[5]
References
edit- ^ a b "Calcipotriol". SPS - Specialist Pharmacy Service. 28 April 2015. Archived from the original on 11 October 2021. Retrieved 29 December 2021.
- ^ a b c d e f g h "Calcipotriene Monograph for Professionals". Drugs.com. Archived from the original on 26 July 2017. Retrieved 29 December 2021. Cite error: The named reference "AHFS2021" was defined multiple times with different content (see the help page).
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 452. ISBN 9783527607495. Archived from the original on 2021-01-22. Retrieved 2021-03-03.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ a b "Calcipotriene Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 25 October 2016. Retrieved 29 December 2021.