 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Intelence |
| Other names | TMC125 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608016 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | NNRTI[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 99.9% |
| Metabolism | Liver (CYP3A4, CYP2C9 & CYP2C19-mediated) |
| Elimination half-life | 41±20 hours |
| Excretion | Faeces (93.7%), urine (1.2%) |
| Identifiers | |
| |
| Chemical and physical data | |
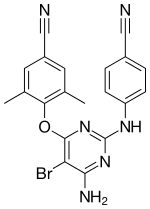
| Formula | C20H15BrN6O |
| Molar mass | 435.285 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Etravirine (ETR) sold under the brand name Intelence, is a medication used to treat and prevent HIV/AIDS.[2] It is used in people who have been previously treated, together with other antiretroviral medications.[1][4] It is taken by mouth twice per day.[2]
Common side effects include rash, diarrhea, nausea, peripheral nerve problems, and headache.[1][2] Other side effects may include Stevens-Johnson syndrome, immune reconstitution syndrome, and lipodystrophy.[2] Safety in pregnancy is unclear.[2] It is a non-nucleoside reverse transcriptase inhibitor (NNRTI), which blocks reverse transcriptase.[1]
Etravirine was approved for medical use in the Canada, Europe, and the United States in 2008.[2][5][1] It is available as a generic medication.[6] In the United Kingdom a month of treatment costs the NHS about £300 as of 2021.[3] This amount in the United States costs about 380 USD.[6]
References
edit- ^ a b c d e f "Intelence". Archived from the original on 18 November 2021. Retrieved 16 December 2021.
- ^ a b c d e f g h "Etravirine Monograph for Professionals". Drugs.com. Archived from the original on 31 July 2020. Retrieved 16 December 2021.
- ^ a b BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 684. ISBN 978-0857114105.
- ^ "Etravirine" (PDF). March 2009. Archived (PDF) from the original on 15 April 2021. Retrieved 16 December 2021.
- ^ "Newsletter July 2008". pmprb-cepmb.gc.ca. 20 June 2014. Archived from the original on 18 October 2020. Retrieved 16 December 2021.
- ^ a b "Etravirine Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 5 November 2016. Retrieved 16 December 2021.