 | |
| Clinical data | |
|---|---|
| Trade names | Decapeptyl, Gonapeptyl, Triptodur, others |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | IM |
| Drug class | GnRH analogue; GnRH agonist; antigonadotropin |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Excretion | Kidney |
| Identifiers | |
| |
| Chemical and physical data | |
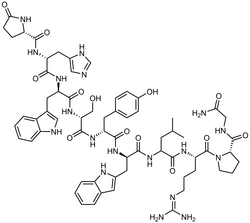
| Formula | C64H82N18O13 |
| Molar mass | 1311.473 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Triptorelin, sold under the brand names Decapeptyl among others, is a medication used for endometriosis, fibroids, prostatic cancer, precocious puberty, and to male hypersexuality with severe sexual deviation.[1][2] It has also been used to delay puberty in people with gender dysphoria.[3] It is given by injection into a muscle.[1]
Common side effects include flushing, sexual dysfunction, pain at the site of injection, and high blood sugar.[1] Other side effects may include pituitary apoplexy, irritability, blood clots, and anaphylaxis.[1] Use during pregnancy may harm the baby.[1] It is a gonadotropin-releasing hormone which decreases the production of androgens and estrogen.[2]
Triptorelin was patented in 1975 and approved for medical use in 1986.[4] It is on the World Health Organization's List of Essential Medicines as an alternative to leuprorelin.[5] In the United Kingdom a 3.75 mg dose costs the NHS about £82 as of 2021.[2] This amount in the United States costs about 860 USD.[6]
References
edit- ^ a b c d e f g "Triptorelin Monograph for Professionals". Drugs.com. Archived from the original on 27 January 2021. Retrieved 20 September 2021.
- ^ a b c d BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 781. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ Barnes, Hannah; Cohen, Deborah (2019-09-20). "Gender dysphoria in children: puberty blockers study draws further criticism". BMJ. 366: l5647. doi:10.1136/bmj.l5647. ISSN 0959-8138. PMID 31540909. S2CID 202711942. Archived from the original on 2021-06-16. Retrieved 2021-07-14.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 514. ISBN 9783527607495. Archived from the original on 2021-06-20. Retrieved 2021-07-14.
- ^ World Health Organization (2023). The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. hdl:10665/371090. WHO/MHP/HPS/EML/2023.02.
- ^ "Trelstar Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 19 April 2021. Retrieved 20 September 2021.