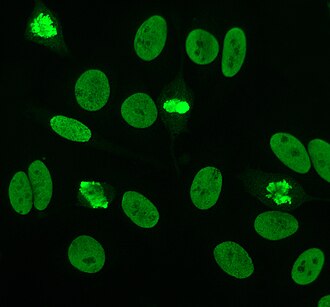
Anti-nuclear antibodies (ANAs, also known as anti-nuclear factor or ANF)[1] are autoantibodies that bind to contents of the cell nucleus. In normal individuals, the immune system produces antibodies to foreign proteins (antigens) but not to human proteins (autoantigens). In some individuals, antibodies to human antigens are produced.[2]
There are many subtypes of ANAs such as anti-Ro antibodies, anti-La antibodies, anti-Sm antibodies, anti-nRNP antibodies, anti-Scl-70 antibodies, anti-dsDNA antibodies, anti-histone antibodies, antibodies to nuclear pore complexes, anti-centromere antibodies and anti-sp100 antibodies. Each of these antibody subtypes bind to different proteins or protein complexes within the nucleus. They are found in many disorders including autoimmunity, cancer and infection, with different prevalences of antibodies depending on the condition. This allows the use of ANAs in the diagnosis of some autoimmune disorders, including systemic lupus erythematosus, Sjögren's syndrome, Scleroderma, polymyositis, dermatomyositis, autoimmune hepatitis and drug induced lupus.[3]
The ANA test detects the autoantibodies present in an individuals blood serum. The common tests used for detecting and quantifying ANAs are indirect immunofluorescence and enzyme-linked immunosorbent assay (ELISA). In immunofluorescence, the level of antibodies are referred to as a titre, which is the point the serum is still positive when diluted. Clinically significant titres are usually considered as 1:160 or more, as titres of less than 1:160 are present in up to 20% of the healthy population, especially the elderly. Titres of 1:160 or higher are only found in 5% of healthy individuals and are more strongly linked with autoimmune disorders. Antibody levels are important in diagnosing autoimmune disorders and predicting the progression of the disease.[4][3][5]
Immunity and autoimmunity
editThe human body has many defence mechanisms against pathogens, one of which is humoral immunity. This defence mechanism produces antibodies (large glycoproteins) in response to an immune stimulus. Many cells of the immune system are required for this process, including lymphocytes (T-cells and B-cells) and antigen presenting cells. These cells coordinate an immune response upon the detection of foreign proteins (antigens), producing antibodies that bind to these antigens. In normal physiology, lymphocytes that recognise human proteins (autoantigens) either undergo programmed cell death (apoptosis) or become non-functional. This self-tolerance means that lymphocytes should not incite an immune response against human cellular antigens. Sometimes, however, this process malfunctions and antibodies are produced against human antigens, which may lead to autoimmune disease.[2]
ANA subtypes
editANAs are found in many disorders, as well as some healthy individuals. These disorders include: systemic lupus erythematosus (SLE), rheumatoid arthritis, Sjögren's syndrome, scleroderma, polymyositis, dermatomyositis, primary biliary cirrhosis, drug induced lupus, Raynaud's phenomenon, autoimmune hepatitis, multiple sclerosis, discoid lupus, thyroid disease, fibromyalgia, antiphospholipid syndrome, juvenile idiopathic arthritis, psoriatic arthritis, juvenile dermatomyositis, idiopathic thrombocytopaenic purpura, infection and cancer. These antibodies can be subdivided according to their specificity, with each subset having differing propensities for specific disorders.[3][6]
Extractable nuclear antigens
editExtractable nuclear antigens (ENA) are a group of autoantigens that were originally identified as antibody targets in people with autoimmune disorders. They are termed ENA because they can be extracted from the cell nucleus with saline.[3][7] The ENAs consist of ribonucleoproteins and non-histone proteins, named by either the name of the donor who provided the prototype serum (Sm, Ro, La, Jo), or the name of the disease setting in which the antibodies were found (SS-A, SS-B, Scl-70).[8]
Anti-Ro/SS-A and anti-La/SS-B
editAnti-Ro and anti-La antibodies, also known as SS-A and SS-B, respectively, are commonly found in primary Sjögren's syndrome; an autoimmune disorder that affects the exocrine glands. The presence of both antibodies are found in 30–60% of Sjögren's syndrome, anti-Ro antibodies alone are found in 50–70% of Sjögren's syndrome and 30% of SLE with cutaneous involvement, and anti-La antibodies are rarely found in isolation.[4][9] Anti-La antibodies are also found in SLE, however Sjögren's syndrome is normally also present.[10] Anti-Ro antibodies are also found less frequently in other disorders including autoimmune liver diseases, coeliac disease, autoimmune rheumatic diseases, cardiac neonatal lupus erythematosus and polymyositis.[11][12] During pregnancy, anti-Ro antibodies can cross the placenta and cause neonatal lupus in babies.[13] In Sjögren's syndrome, anti-Ro and anti-La antibodies correlate with early onset, increased disease duration, parotid gland enlargement, disease outside the glands and infiltration of glands by lymphocytes.[5] Anti-Ro antibodies are specific to components of the Ro-RNP complex, comprising 45kDa, 52kDa, 54kDa and 60kDa proteins and RNA. The 60kDa DNA/RNA binding protein and 52kDa T-cell regulatory protein are the best characterised antigens of anti-Ro antibodies. Collectively, these proteins are part of a ribonucleoprotein (RNP) complex that associate with the hyRNAs, hY1-hY5. The La antigen is a 48kDa transcription termination factor of RNA polymerase III, which associates with the Ro-RNP complex.[8][9][14][15]
The mechanism of antibody production in Sjögren's syndrome is not fully understood, but apoptosis (programmed cell death) and molecular mimicry may play a role.[5] The Ro and La antigens are expressed on the surface of cells undergoing apoptosis and may cause the inflammation within the salivary gland by interaction with cells of the immune system. The antibodies may also be produced through molecular mimicry, where cross reactive antibodies bind to both virus and human proteins. This may occur with one of the antigens, Ro or La, and may subsequently produce antibodies to other proteins through a process known as epitope spreading. The retroviral gag protein shows similarity to the La protein and is proposed as a possible example for molecular mimicry in Sjögren's syndrome.[5][12]
Anti-Sm
editAnti-Smith (Anti-Sm) antibodies are a very specific marker for SLE. Approximately 99% of individuals with anti-Sm antibodies have the disease, but only 20% of people with SLE have the antibodies. They are associated with central nervous system involvement, kidney disease, lung fibrosis and pericarditis in SLE, but they are not associated with disease activity. The antigens of the anti-Sm antibodies are the core units of the small nuclear ribonucleoproteins (snRNPs), termed A to G, and will bind to the U1, U2, U4, U5 and U6 snRNPs. Most commonly, the antibodies are specific for the B, B' and D units.[16][17] Molecular and epidemiological studies suggest that anti-Sm antibodies may be induced by molecular mimicry because the protein shows some similarity to Epstein-Barr virus proteins.[18][19]
Anti-nRNP/anti-U1-RNP
editAnti-nuclear ribonucleoprotein (anti-nRNP) antibodies, also known as anti-U1-RNP antibodies, are found in 30–40% of SLE. They are often found with anti-Sm antibodies, but they may be associated with different clinical associations. In addition to SLE, these antibodies are highly associated with mixed connective tissue disease. Anti-nRNP antibodies recognise the A and C core units of the snRNPs and because of this they primarily bind to the U1-snRNP.[16][20] The immune response to RNP may be caused by the presentation of the nuclear components on the cell membrane in apoptotic blebs. Molecular mimicry has also been suggested as a possible mechanism for the production of antibodies to these proteins because of similarity between U1-RNP polypeptides and Epstein-Barr virus polypeptides.[21]
Anti-Scl-70/anti-topoisomerase I
editAnti-Scl-70 antibodies are linked to scleroderma.[22] The sensitivity of the antibodies for scleroderma is approximately 34%, but is higher for cases with diffuse cutaneous involvement (40%), and lower for limited cutaneous involvement (10%). The specificity of the antibodies is 98% and 99.6% in other rheumatic diseases and normal individuals, respectively.[3][23] In addition to scleroderma, these antibodies are found in approximately 5% of individuals with SLE.[24] The antigenic target of anti-Scl-70 antibodies is topoisomerase I.[25]
Anti-Jo-1
editAlthough anti-Jo-1 antibodies are often included with ANAs, they are actually antibodies to the cytoplasmic protein, histidyl tRNA sythetase.[7] They are highly associated with polymyositis and dermatomyositis, and are rarely found in other connective tissue diseases. Around 20–40% of polymyositis is positive for Jo-1 antibodies and most will have interstitial lung disease, HLA-DR3 and HLA-DRw52 human leukocyte antigen (HLA) markers; collectively known as Jo-1 syndrome.[16][26]
Anti-dsDNA
editAnti-double stranded DNA (anti-dsDNA) antibodies are highly associated with SLE. They are a very specific marker for the disease, with some studies quoting nearly 100%.[3] Data on sensitivity ranges from 25–85%. Anti-dsDNA antibody levels, known as titres, correlate with disease activity in SLE; high levels indicate more active lupus. The presence of anti-dsDNA antibodies is also linked with lupus nephritis and there is evidence they are the cause. Some anti-dsDNA antibodies are cross reactive with other antigens found on the glomerular basement membrane (GBM) of the kidney, such as heparan sulphate, collagen IV, fibronectin and laminin. Binding to these antigens within the kidney could cause inflammation and complement fixation, resulting in kidney damage. It is also possible that the anti-dsDNA antibodies are internalised by cells when they bind membrane antigens and then are displayed on the cell surface. This could promote inflammatory responses by T-cells within the kidney. It is important to note that not all anti-dsDNA antibodies are associated with lupus nephritis and that other factors can cause this symptom in their absence. The antigen of anti-dsDNA antibodies is double stranded DNA.[27][28]
Anti-histone antibodies
editAnti-histone antibodies are found in the serum of up to 75–95% of people with drug induced lupus and 75% of idiopathic SLE. Unlike anti-dsDNA antibodies in SLE, these antibodies do not fix complement. Although they are most commonly found in drug induced lupus, they are also found in some cases of SLE, scleroderma, rheumatoid arthritis and undifferentiated connective tissue disease. Many drugs are known to cause drug induced lupus and they produce various antigenic targets within the nucleosome that are often cross reactive with several histone proteins and DNA. Procainamide causes a form of drug-induced lupus that produces antibodies to the histone H2A and H2B complex.[29][30]
Anti-gp210 and anti-p62
editBoth anti-glycoprotein-210 (anti-gp210) and anti-nucleoporin 62 (anti-p62) antibodies are antibodies to components of the nuclear membrane and are found in primary biliary cirrhosis (PBC). Each antibody is present in approximately 25–30% of PBC. The antigens of both antibodies are constituents of the nuclear membrane. gp210 is a 200kDa protein involved in anchoring components of the nuclear pore to the nuclear membrane. The p62 antigen is a 60kDa nuclear pore complex.[31][32]
Anti-centromere antibodies
editAnti-centromere antibodies are associated with limited cutaneous systemic sclerosis, also known as CREST syndrome, primary biliary cirrhosis and proximal scleroderma.[33] There are six known antigens, which are all associated with the centromere; CENP-A to CENP-F. CENP-A is a 17kDa histone H3-like protein. CENP-B is an 80kDa DNA binding protein involved in the folding of heterochromatin. CENP-C is a 140kDa protein involved in kinetochore assembly. CENP-D is a 50kDa protein of unknown function, but may be homologous to another protein involved in chromatin condensation, RCC1. CENP-E is a 312kDa protein from the kinesin motor protein family. CENP-F is a 367kDa protein from the nuclear matrix that associates with the kinetochore in late G2 phase during mitosis. CENP-A, B and C antibodies are most commonly found (16–42% of systemic sclerosis) and are associated with Raynaud's phenomenon, telangiectasias, lung involvement and early onset in systemic sclerosis.[23][34][35]
Anti-sp100
editAnti-sp100 antibodies are found in approximately 20–30% of primary biliary cirrhosis (PBC). They are found in few individuals without PBC, and therefore are a very specific marker of the disease. The sp100 antigen is found within nuclear bodies; large protein complexes in the nucleus that may have a role in cell growth and differentiation.[36]
Anti-PM-Scl
editAnti-PM-Scl antibodies are found in up to 50% of polymyositis/systemic scleorosis (PM/SSc) overlap syndrome. Around 80% of individuals with antibodies present in their blood serum will have the disorder. The presence of the antibodies is linked to limited cutaneous involvement of PM/SSc overlap syndrome. The antigenic targets of the antibodies are components of the RNA-processing exosome complex in the nucleolus.[23] There are ten proteins in this complex and antibodies to eight of them are found at varying frequencies; PM/Scl-100 (70–80%), PM/Scl-75 (46–80%), hRrp4 (50%), hRrp42 (21%), hRrp46 (18%), hCs14 (14%), hRrp41 (10%) and hRrp40 (7%).[37]
ANA test
editThe presence of ANAs in blood can be confirmed by a screening test. Although there are many tests for the detection of ANAs, the most common tests used for screening are indirect immunofluoresence and enzyme-linked immunosorbent assay (ELISA).[38] Following detection of ANAs, various subtypes are determined.[3]
Indirect immunofluorescence
editIndirect immunofluorescence is one of the most commonly used tests for ANAs. Typically, HEp-2 cells are used as a substrate to detect the antibodies in human serum. Microscope slides are coated with HEp-2 cells and the serum is incubated with the cells. If antibodies are present then they will bind to the antigens on the cells; in the case of ANAs, the antibodies will bind to the nucleus. These can be visualised by adding a fluorescent tagged (usually FITC or rhodopsin B) anti-human antibody that binds to the antibodies. The molecule will fluoresce when a specific wavelength of light shines on it, which can be seen under the microscope. Depending on the antibody present in the human serum and the localisation of the antigen in the cell, distinct patterns of fluorescence will be seen on the HEp-2 cells.[39][40] Levels of antibodies are analysed by performing dilutions on blood serum. An ANA test is considered positive if fluorescence is seen at a titre of 1:40/1:80. Higher titres are more clinically significant as low positives (≤1:160) are found in up to 20% of healthy individuals, especially the elderly. Only around 5% of the healthy population have ANA titres of 1:160 or higher.[3][41]
HEp-2
editUntil around 1975, when HEp-2 cells were introduced, animal tissue was used as the standard substrate for immunofluorescence.[4] HEp-2 cells are currently one of the most common substrates for ANA detection by immunofluorescence. They are superior to the previously used animal tissues because of their large size and the high rate of mitosis (cell division) in the cell line. This allows the detection of antibodies to mitosis-specific antigens, such as centromere antibodies. They also allow identification of anti-Ro antibodies, because acetone is used for fixation of the cells (other fixatives can wash the antigen away).[42]
There are many nuclear staining patterns seen on HEp-2 cells; homogeneous, speckled, nucleolar, nuclear membranous, centromeric, nuclear dot and pleiomorphic. The homogeneous pattern is seen when the condensed chromosomes and interphase chromatin stain. This pattern is associated with anti-dsDNA antibodies, antibodies to nucleosomal components and anti-histone antibodies. There are two speckled patterns; fine and coarse. The fine speckled pattern has fine nuclear staining with unstained metaphase chromatin, which is associated with anti-Ro and anti-La antibodies. The coarse staining pattern has coarse granular nuclear staining, caused by anti-U1-RNP and anti-Sm antibodies. The nucleolar staining pattern is associated with many antibodies including anti-Scl-70, anti-PM-Scl, anti-fibrillarin and anti-Th/To. Nuclear membrane staining appears as a fluorescent ring around the cell nucleus and are produced by anti-gp210 and anti-p62 antibodies. The centromere pattern shows multiple nuclear dots in interphase and mitotic cells, corresponding to the number of chromosomes in the cell. Nuclear dot patterns show between 13–25 nuclear dots in interphase cells and are produced by anti-sp100 antibodies. Pleiomorphic pattern is caused by antibodies to the proliferating cell nuclear antigen.[16][41][43][44]
Crithidia luciliae
editCrithidia luciliae are haemoflaggelate single celled protists. They are used as a substrate in immunofluorescence for the detection of anti-dsDNA antibodies. They possess an organelle known as the kinetoplast which is a large mitochondrion with a network of interlocking circular dsDNA molecules. After incubation with serum containing anti-dsDNA antibodies and fluorescent-labelled anti-human antibodies, the kinetoplast will fluoresce. The lack of other nuclear antigens in this organelle means that using C.luciliae as a substrate allows for the specific detection of anti-dsDNA antibodies.[3][45][46]
ELISA
editEnzyme-linked immunosorbent assay (ELISA) uses antigen-coated microtitre plates for the detection of ANAs. Each well of a microtitre plate is coated with either a single antigen or multiple antigens to detect specific antibodies or to screen for ANAs, respectively. The antigens are either from cell extracts or recombinant. Blood serum is incubated in the wells of the plate and is washed out. If antibodies that bind to antigen are present then they will remain after washing. A secondary anti-human antibody conjugated to an enzyme such as horseradish peroxidase. The enzyme reaction will produce a change in colour of the solution that is proportional to the amount of antibody bound to the antigen.[4][40][47]
Sensitivity
editThe following table list the prevalence of different types of ANAs for different diseases, in this case what percentage of those with the disease have the ANA. Some ANAs appear in several types of disease, resulting in lower specificity of the test.
| ANA type | Target antigen | Sensitivity (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| SLE | Drug-induced LE | Diffuse systemic sclerosis | Limited systemic scleroderma | Sjögren syndrome | Inflammatory myopathy | MCTD | ||
| All ANAs (by indirect IF) |
Various | >95 | >95 | 70–90 | 70–90 | 50–80 | 40–60 | 95[48] |
| Anti-dsDNA | DNA | 40–60 | – | – | – | – | – | -[48] |
| Anti-Sm | Core proteins of snRNPs | 20–30 | – | – | – | – | – | -[48] |
| Anti-histone | Histones | 50–70 | 90[48] – 95 | – | – | – | – | -[48] |
| Anti Scl-70 | Type I topoisomerase | – | – | 28–70 | 10–18 | – | – | -[48] |
| Anti-centromere | Centromeric proteins | – | – | 22–26 | 90 | – | – | -[48] |
| Anti-snRNP70 | snRNP70 | 30[49]-40[48][49] | -[48] | 15[48] | 10[48] | -[48] | 15[48] | 90[48] |
| SS-A (Ro) | RNPs | 30–50 | – | – | – | 70–95 | 10 | -[48] |
| SS-B (La) | RNPs | 10–15 | – | – | – | 60–90 | – | -[48] |
| Jo-1 | Histidine-tRNA ligase | – | – | – | – | – | 25 | -[48] |
| – = less than 5% sensitivity
Unless else specified in boxes, then ref is:[49] | ||||||||
History
editThe LE cell was discovered in bone marrow in 1948 by Hargraves et al.[50] This was the first indication that processes affecting the cell nucleus were responsible for SLE. In 1959 it was discovered that serum from individuals with SLE contained antibodies that precipitated with saline extracts of nuclei, known as extractable nuclear antigens (ENAs). This led to the characterisation of ENA antigens and their respective antibodies. Thus, anti-Sm and anti-RNP antibodies were discovered in 1966 and 1971, respectively. In the 1970s, the anti-Ro/anti-SS-A and anti-La/anti-SS-B antibodies were discovered. The Scl-70 antibody was known to be a specific antibody to scleroderma in 1979, however the antigen (topoisomerase-I) was not characterised until 1986. The Jo-1 antigen and antibody were characterised in 1980.[3][12]
See also
editReferences
edit- ^ "Medical Subject Headings (MeSH)". Retrieved 12 February 2013.
{{cite web}}:|first=missing|last=(help) - ^ a b Reece, Neil A. Campbell, Jane B. (2005). Biology (7th ed.). San Francisco: Pearson/Benjamin-Cummings. ISBN 080537146X.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j Kavanaugh A, Tomar R, Reveille J, Solomon DH, Homburger HA. Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. American College of Pathologists. Arch Pathol Lab Med 2000;124:71–81. PMID 10629135.
- ^ a b c d Kumar, Y.; Bhatia, A.; Minz, R. W. (2009 Jan 2). "Antinuclear antibodies and their detection methods in diagnosis of connective tissue diseases: a journey revisited". Diagnostic Pathology. 4: 1. doi:10.1186/1746-1596-4-1. PMC 2628865. PMID 19121207.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: unflagged free DOI (link) - ^ a b c d Yamamoto K (January 2003). "Pathogenesis of Sjögren's syndrome". Autoimmun Rev. 2 (1): 13–8. doi:10.1016/s1568-9972(02)00121-0. PMID 12848970.
{{cite journal}}: CS1 maint: date and year (link) - ^ Malleson PN, Mackinnon MJ, Sailer-Hoeck M, Spencer CH (2010). "Review for the generalist: The antinuclear antibody test in children – When to use it and what to do with a positive titer". Pediatr Rheumatol Online J. 8: 27. doi:10.1186/1546-0096-8-27. PMC 2987328. PMID 20961429.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b Damoiseaux, J. G.; Tervaert, J. W. (2006 Jan). "From ANA to ENA: how to proceed?". Autoimmunity Reviews. 5 (1): 10–7. doi:10.1016/j.autrev.2005.05.007. PMID 16338206.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Wenzel, J.; Gerdsen, R.; Uerlich, M.; Bauer, R.; Bieber, T.; Boehm, I. (2001 Dec). "Antibodies targeting extractable nuclear antigens: historical development and current knowledge". The British Journal of Dermatology. 145 (6): 859–67. doi:10.1046/j.1365-2133.2001.04577.x. PMID 11899137.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Hernández-Molina, G.; Leal-Alegre, G.; Michel-Peregrina, M. (2011 Jan). "The meaning of anti-Ro and anti-La antibodies in primary Sjögren's syndrome". Autoimmunity Reviews. 10 (3): 123–5. doi:10.1016/j.autrev.2010.09.001. PMID 20833272.
{{cite journal}}: Check date values in:|date=(help) - ^ Kassan, S. S.; Moutsopoulos, H. M. (2004 Jun). "Clinical manifestations and early diagnosis of Sjögren syndrome". Arch Intern Med. 164 (12): 1275–84. doi:10.1001/archinte.164.12.1275. PMID 15226160.
{{cite journal}}: Check date values in:|date=(help) - ^ Defendenti, C.; Atzeni, F.; Spina, M. F.; Grosso, S.; Cereda, A.; Guercilena, G.; Bollani, S.; Saibeni, S.; Puttini, P. S. (2011 Jan). "Clinical and laboratory aspects of Ro/SSA-52 autoantibodies". Autoimmunity Reviews. 10 (3): 150–4. doi:10.1016/j.autrev.2010.09.005. PMID 20854935.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c Venables, PJ (2004 Jun). "Sjögren's syndrome". Best Practice & Research. Clinical Rheumatology. 18 (3): 313–29. doi:10.1016/j.berh.2004.02.010. PMID 15158743.
{{cite journal}}: Check date values in:|date=(help) - ^ Scofield, RH (2004 May 8). "Autoantibodies as predictors of disease". Lancet. 363 (9420): 1544–6. doi:10.1016/S0140-6736(04)16154-0. PMID 15135604.
{{cite journal}}: Check date values in:|date=(help) - ^ Deshmukh, U. S.; Bagavant, H.; Lewis, J.; Gaskin, F.; Fu, S. M. (2005 Nov). "Epitope spreading within lupus-associated ribonucleoprotein antigens". Clinical Immunology (Orlando, Fla.). 117 (2): 112–20. doi:10.1016/j.clim.2005.07.002. PMID 16095971.
{{cite journal}}: Check date values in:|date=(help) - ^ Ben-Chetrit, E (1993 May). "The molecular basis of the SSA/Ro antigens and the clinical significance of their autoantibodies". British Journal of Rheumatology. 32 (5): 396–402. doi:10.1093/rheumatology/32.5.396. PMID 8495261.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c d von Mühlen, C. A.; Tan, E. M. (1995 Apr). "Autoantibodies in the diagnosis of systemic rheumatic diseases". Seminars in Arthritis and Rheumatism. 24 (5): 323–58. doi:10.1016/s0049-0172(95)80004-2. PMID 7604300.
{{cite journal}}: Check date values in:|date=(help) - ^ Lyons, R.; Narain, S.; Nichols, C.; Satoh, M.; Reeves, W. H. (2005 Jun). "Effective use of autoantibody tests in the diagnosis of systemic autoimmune disease". Annals of the New York Academy of Sciences. 1050: 217–28. doi:10.1196/annals.1313.023. PMID 16014537.
{{cite journal}}: Check date values in:|date=(help) - ^ Zieve, G. W.; Khusial, P. R. (2003 Sep). "The anti-Sm immune response in autoimmunity and cell biology". Autoimmunity Reviews. 2 (5): 235–40. doi:10.1016/s1568-9972(03)00018-1. PMID 12965173.
{{cite journal}}: Check date values in:|date=(help) - ^ Migliorini, P.; Baldini, C.; Rocchi, V.; Bombardieri, S. (2005 Feb). "Anti-Sm and anti-RNP antibodies". Autoimmunity. 38 (1): 47–54. doi:10.1080/08916930400022715. PMID 15804705.
{{cite journal}}: Check date values in:|date=(help) - ^ Benito-Garcia, E.; Schur, P. H.; Lahita, R.; American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines (2004 Dec 15). "Guidelines for immunologic laboratory testing in the rheumatic diseases: anti-Sm and anti-RNP antibody tests". Arthritis and Rheumatism. 51 (6): 1030–44. doi:10.1002/art.20836. PMID 15593352.
{{cite journal}}: Check date values in:|date=(help) - ^ Venables PJ (2006). "Mixed connective tissue disease". Lupus. 15 (3): 132–7. doi:10.1191/0961203306lu2283rr. PMID 16634365.
- ^ Jimenez, SA (2004 Jan 6). "Following the molecular pathways toward an understanding of the pathogenesis of systemic sclerosis". Annals of Internal Medicine. 140 (1): 37–50. doi:10.7326/0003-4819-140-1-200401060-00010. PMID 14706971.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c Ho, K. T.; Reveille, J. D. (2003). "The clinical relevance of autoantibodies in scleroderma". Arthritis Research & Therapy. 5 (2): 80–93. doi:10.1186/ar628. PMC 165038. PMID 12718748.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Mahler M, Silverman ED, Schulte-Pelkum J, Fritzler MJ (September 2010). "Anti-Scl-70 (topo-I) antibodies in SLE: Myth or reality?". Autoimmun Rev. 9 (11): 756–60. doi:10.1016/j.autrev.2010.06.005. PMID 20601198.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Guldner, H. H.; Szostecki, C.; Vosberg, H. P.; Lakomek, H. J.; Penner, E.; Bautz, F. A. (1986). "Scl 70 autoantibodies from scleroderma patients recognize a 95 kDa protein identified as DNA topoisomerase I.". Chromosoma. 94 (2): 132–8. doi:10.1007/BF00286991. PMID 2428564.
- ^ Schmidt, W. A.; Wetzel, W.; Friedländer, R.; Lange, R.; Sörensen, H. F.; Lichey, H. J.; Genth, E.; Mierau, R.; Gromnica-Ihle, E. (2000). "Clinical and serological aspects of patients with anti-Jo-1 antibodies—an evolving spectrum of disease manifestations". Clinical Rheumatology. 19 (5): 371–7. doi:10.1007/s100670070030. PMID 11055826.
- ^ Mok, C. C.; Lau, C. S. (2003 Jul). "Pathogenesis of systemic lupus erythematosus". Journal of Clinical Pathology. 56 (7): 481–90. doi:10.1136/jcp.56.7.481. PMC 1769989. PMID 12835292.
{{cite journal}}: Check date values in:|date=(help) - ^ Yung, S.; Chan, T. M. (2008 Feb). "Anti-DNA antibodies in the pathogenesis of lupus nephritis—the emerging mechanisms". Autoimmunity Reviews. 7 (4): 317–21. doi:10.1016/j.autrev.2007.12.001. PMID 18295737.
{{cite journal}}: Check date values in:|date=(help) - ^ Vasoo, S (2006). "Drug-induced lupus: an update". Lupus. 15 (11): 757–61. doi:10.1177/0961203306070000. PMID 17153847.
- ^ Katz, U.; Zandman-Goddard, G. (2010 Nov). "Drug-induced lupus: an update". Autoimmunity Reviews. 10 (1): 46–50. doi:10.1016/j.autrev.2010.07.005. PMID 20656071.
{{cite journal}}: Check date values in:|date=(help) - ^ Hu, T.; Guan, T.; Gerace, L. (1996 Aug). "Molecular and functional characterization of the p62 complex, an assembly of nuclear pore complex glycoproteins". The Journal of Cell Biology. 134 (3): 589–601. doi:10.1083/jcb.134.3.589. PMC 2120945. PMID 8707840.
{{cite journal}}: Check date values in:|date=(help) - ^ MacKay, I. R.; Whittingham, S.; Fida, S.; Myers, M.; Ikuno, N.; Gershwin, M. E.; Rowley, M. J. (2000 Apr). "The peculiar autoimmunity of primary biliary cirrhosis". Immunological Reviews. 174: 226–37. doi:10.1034/j.1600-0528.2002.017410.x. PMID 10807519.
{{cite journal}}: Check date values in:|date=(help) - ^ Kallenberg, CG (1990 Mar). "Anti-centromere antibodies (ACA)". Clinical Rheumatology. 9 (1 Suppl 1): 136–9. doi:10.1007/BF02205562. PMID 2203592.
{{cite journal}}: Check date values in:|date=(help) - ^ Rattner, J. B.; Mack, G. J.; Fritzler, M. J. (1998 Jul). "Autoantibodies to components of the mitotic apparatus". Molecular Biology Reports. 25 (3): 143–55. doi:10.1023/a:1016523013819. PMID 9700050.
{{cite journal}}: Check date values in:|date=(help) - ^ Renz, Harald (2012). Autoimmune diagnostics. Berlin: De Gruyter. ISBN 978-3-11-022864-9.
- ^ Worman, H. J.; Courvalin, J. C. (2003 Jun). "Antinuclear antibodies specific for primary biliary cirrhosis". Autoimmunity Reviews. 2 (4): 211–7. doi:10.1016/s1568-9972(03)00013-2. PMID 12848948.
{{cite journal}}: Check date values in:|date=(help) - ^ Mahler, M.; Raijmakers, R. (2007 Aug). "Novel aspects of autoantibodies to the PM/Scl complex: clinical, genetic and diagnostic insights". Autoimmunity Reviews. 6 (7): 432–7. doi:10.1016/j.autrev.2007.01.013. PMID 17643929.
{{cite journal}}: Check date values in:|date=(help) - ^ Granito A, Muratori P, Quarneti C, Pappas G, Cicola R, Muratori L (January 2012). "Antinuclear antibodies as ancillary markers in primary biliary cirrhosis". Expert Review of Molecular Diagnostics. 12 (1): 65–74. doi:10.1586/erm.11.82. PMID 22133120.
{{cite journal}}: CS1 maint: date and year (link) CS1 maint: multiple names: authors list (link) - ^ Klein, Wulf B. Storch. Transl. by R. A. (2000). Immunofluorescence in clinical immunology : a primer and atlas. Basel [u.a.]: Birkhäuser. ISBN 3764361824.
- ^ a b González-Buitrago, J. M.; González, C. (2006 Mar). "Present and future of the autoimmunity laboratory". Clinica Chimica Acta; International Journal of Clinical Chemistry. 365 (1–2): 50–7. doi:10.1016/j.cca.2005.07.023. PMID 16126186.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Tozzoli, R.; Bizzaro, N.; Tonutti, E.; Villalta, D.; Bassetti, D.; Manoni, F.; Piazza, A.; Pradella, M.; Rizzotti, P.; Italian Society of Laboratory Medicine Study Group on the Diagnosis of Autoimmune Diseases (2002 Feb). "Guidelines for the laboratory use of autoantibody tests in the diagnosis and monitoring of autoimmune rheumatic diseases". American Journal of Clinical Pathology. 117 (2): 316–24. doi:10.1309/Y5VF-C3DM-L8XV-U053. PMID 11863229.
{{cite journal}}: Check date values in:|date=(help) - ^ Keren, DF (2002 Jun). "Antinuclear antibody testing". Clinics in Laboratory Medicine. 22 (2): 447–74. doi:10.1016/s0272-2712(01)00012-9. PMID 12134471.
{{cite journal}}: Check date values in:|date=(help) - ^ Nesher, G.; Margalit, R.; Ashkenazi, Y. J. (2001 Apr). "Anti-nuclear envelope antibodies: Clinical associations". Seminars in Arthritis and Rheumatism. 30 (5): 313–20. doi:10.1053/sarh.2001.20266. PMID 11303304.
{{cite journal}}: Check date values in:|date=(help) - ^ Sack, U (2009 Jun). "Autoantibody detection by indirect immunofluorescence on HEp-2 cells". Deutsche medizinische Wochenschrift (1946). 134 (24): 1278–82. doi:10.1055/s-0029-1225278. PMID 19499499.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Slater, N. G.; Cameron, J. S.; Lessof, M. H. (1976 Sep). "The Crithidia luciliae kinetoplast immunofluorescence test in systemic lupus erythematosus". Clinical and Experimental Immunology. 25 (3): 480–6. PMC 1541410. PMID 786521.
{{cite journal}}: Check date values in:|date=(help) - ^ Shapiro, TA (1995). "The structure and replication of kinetoplast DNA". Annual Review of Microbiology. 49: 117–43. doi:10.1146/annurev.mi.49.100195.001001. PMID 8561456.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Deshpande, S. S. (1996). Enzyme immunoassays: from concept to product development. London: Chapman & Hall. ISBN 0-412-05601-1.
- ^ a b c d e f g h i j k l m n o p q Table 6-2 in: Elizabeth D Agabegi; Agabegi, Steven S. (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 978-0-7817-7153-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c Table 5-9 in: Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson (2007). Robbins Basic Pathology. Philadelphia: Saunders. ISBN 978-1-4160-2973-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) 8th edition. - ^ Hargraves M, Richmond H, Morton R. Presentation of two bone marrow components, the tart cell and the LE cell. Mayo Clin Proc 1948;27:25–28.
External links
edit- Site with unique immunofluorescence images and slides -organ and non-organ specific
- Antinuclear+antibodies at the U.S. National Library of Medicine Medical Subject Headings (MeSH)