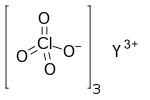
Yttrium perchlorate is the inorganic compound with the chemical formula Y(ClO
4)
3.[1][2] The compound is an yttrium salt of perchloric acid.[3][4]
| |
| Names | |
|---|---|
| Other names
Yttrium triperchlorate, yttrium(III) perchlorate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.034.388 |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| Y(ClO 4) 3 | |
| Molar mass | 387.244 |
| Appearance | liquid |
| Density | g/cm–3 |
| soluble | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Oxidizer |
| GHS labelling: | |
 
| |
| Danger | |
| P210, P220, P260, P264, P280, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P321, P363, P370+P378, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
editDissolving yttrium oxide in perchloric acid solution can produce yttrium perchlorate octahydrate.[citation needed]
Chemical properties
editPotentially explosive.[5]
Physical properties
editThe compound is soluble in water and forms a hexahydrate with the formula Y(ClO
4)
3•6H
2O.[6][7]
References
edit- ^ "Yttrium(III) Perchlorate Solution". American Elements. Retrieved 14 March 2023.
- ^ "CAS 14017-56-2 Yttrium perchlorate - Alfa Chemistry". alfa-chemistry.com. Retrieved 14 March 2023.
- ^ "Yttrium(III) perchlorate, 50% w/w aq. soln., Reagent Grade, Thermo Scientific". Fisher Scientific. Retrieved 14 March 2023.
- ^ Macintyre, Jane E. (13 November 1994). Dictionary of Inorganic Compounds, Supplement 2. CRC Press. p. 585. ISBN 978-0-412-49100-9. Retrieved 15 March 2023.
- ^ Macintyre, Jane E. (23 July 1992). Dictionary of Inorganic Compounds. CRC Press. p. 2931. ISBN 978-0-412-30120-9. Retrieved 15 March 2023.
- ^ "Yttrium Perchlorate, Hydrated, 50% Solution, Reagent". gfschemicals.com. Retrieved 14 March 2023.
- ^ "40580 Yttrium(III) perchlorate, 50% w/w aq. soln., Reagent Grade". Alfa Aesar. Retrieved 14 March 2023.