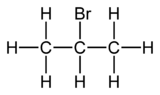
2-Bromopropane, also known as isopropyl bromide and 2-propyl bromide, is the halogenated hydrocarbon with the formula CH3CHBrCH3. It is a colorless liquid. It is used for introducing the isopropyl functional group in organic synthesis. 2-Bromopropane is prepared by heating isopropanol with hydrobromic acid.[3]

| |||
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Bromopropane[2] | |||
| Other names
Isopropyl bromide[1]
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 741852 | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.778 | ||
| EC Number |
| ||
| MeSH | 2-bromopropane | ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2344 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H7Br | |||
| Molar mass | 122.993 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 1.31 g mL−1 | ||
| Melting point | −89.0 °C; −128.1 °F; 184.2 K | ||
| Boiling point | 59 to 61 °C; 138 to 142 °F; 332 to 334 K | ||
| 3.2 g L−1 (at 20 °C) | |||
| log P | 2.136 | ||
| Vapor pressure | 32 kPa (at 20 °C) | ||
Henry's law
constant (kH) |
1.0 μmol Pa−1 mol−1 | ||
Refractive index (nD)
|
1.4251 | ||
| Viscosity | 0.4894 mPa s (at 20 °C) | ||
| Thermochemistry | |||
Heat capacity (C)
|
135.6 J K mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
−129 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
−2.0537–−2.0501 MJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Danger | |||
| H225, H360, H373 | |||
| P210, P308+P313 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 19 °C (66 °F; 292 K) | ||
| Related compounds | |||
Related alkanes
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Preparation
edit2-Bromopropane is commercially available. It may be prepared in the ordinary manner of alkyl bromides, by reacting isopropanol with phosphorus and bromine,[4] or with phosphorus tribromide.[5]
Safety
editShort-chain alkyl halides are often carcinogenic.
The bromine atom is at the secondary position, which allows the molecule to undergo dehydrohalogenation easily to give propene, which escapes as a gas and can rupture closed reaction vessels. When this reagent is used in base catalyzed reactions, potassium carbonate should be used in place of sodium or potassium hydroxide.
Further reading
edit- Max Gergel, “Excuse Me Sir, Would You Like to Buy a Kilo of Isopropyl Bromide?” Pierce Chemical Co. (1979). (story of start-up chemical company).
References
edit- ^ Armarego, Wilfred L.F.; Li Lin Chai, Christina (2013). Purification of laboratory chemicals (7th ed.). Butterworth-Heinemann. p. 176. ISBN 9780123821621.
- ^ "2-bromopropane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 27 March 2005. Identification. Retrieved 15 June 2012.
- ^ "Monograph 6526". Merck Index of Chemicals and Drugs.
- ^ Oliver Kamm and C. S. Marvel (1941). "Alkyl and alkylene bromides". Organic Syntheses; Collected Volumes, vol. 1, p. 25.
- ^ C. R. Noller and R. Dinsmore (1943). "Isobutyl bromide". Organic Syntheses; Collected Volumes, vol. 2, p. 358.


