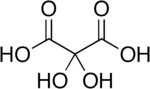
Dihydroxymalonic acid is an organic compound with formula C3H4O6 or HO-(C=O)-C(OH)2-(C=O)-OH, found in some plants such as alfalfa and in beet molasses.[2]
| |
| Names | |
|---|---|
| Preferred IUPAC name
Dihydroxypropanedioic acid | |
| Other names
Mesoxalic acid monohydrate
Oxomalonic acid monohydrate Ketomalonic acid monohydrate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.372 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H4O6 | |
| Molar mass | 136.059 g·mol−1 |
| Melting point | 119 to 120 °C (246 to 248 °F; 392 to 393 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The compound is also called dihydroxymesoxalic acid and dihydroxypropanedioic acid. It can be viewed as a hydrate derivative of mesoxalic acid, and is often called mesoxalic acid monohydrate and similar names.[3] This compound is unusual in containing stable geminal hydroxy groups.
Dihydroxymalonic acid is a water-soluble white solid. It crystallizes in deliquescent prisms that melt between 113 °C and 121 °C without loss of water.[4] It has been used in medical research as a hypoglycemic agent[5] and was patented in the United States in 1997 as a fast-acting antidote to cyanide poisoning.[6]
Synthesis
editDihydroxymalonic acid can be obtained synthetically by hydrolysis of alloxan with baryta water,[2] by warming caffuric acid[7] with lead acetate solution,[4] by electrolysis of tartaric acid in alkaline solution,[8] or from glycerin diacetate and concentrated nitric acid in the cold. The product can be obtained also by oxidation of tartronic acid [9] or glycerol.[10]
Reactions
editLike typical hydrated ketonic acids, it is reduced in aqueous solution by sodium amalgam to tartronic acid, and also combines with phenylhydrazine and hydroxylamine. It reduces ammoniacal silver solutions. When heated with urea to 100 °C, it forms allantoin. By continued boiling of its aqueous solution it is decomposed into carbon dioxide and glyoxylic acid.
See also
editReferences
edit- ^ Merck Index, 12th Edition, 5971.
- ^ a b Deichsel, Theodor (1864). "Ueber die Mesoxalsäure". J. Prakt. Chem. (in German). 93 (1): 193–208. doi:10.1002/prac.18640930139.
- ^ E. T. Urbansky, W. J. Bashe (2000). Journal of Chromatography A, volume 867, pp. 143–149.
- ^ a b Henry Enfield Roscoe (1888), A Treatise on Chemistry, volume 3, part2 Organic Chemistry, p. 161. D. Appleton and Co., New York.
- ^ Yoshito KOBAYASHI, Shigeru OHASHI, Shinzaburo TANAKA and Akitoshi SHIOYA (1955), Hypoglycemic Action of Sodium Mesoxalate with Special Reference to Hyperfunction of Pituitary-Adrenal Cortical System in Dogs Exposed to Cold[permanent dead link]. Proceedings of the Japan Academy, volume 31, issue 8, pp.493–497.
- ^ "Method for the treatment of cyanide poisoning". Archived from the original on 2017-05-11. Retrieved 2009-11-13.
- ^ The chemical structure of caffuric acid was given in Allen, W. F. (1932). The preparation and pyrolytic molecular rearrangment [sic] of the 8-ethers of caffeine: And their conversion to 8-methyl and 8-ethylcaffeine. Ann Arbor, Mich.: Edwards Brothers.
- ^ (1922), Chem. Zentralblatt III, 871
- ^ Ciriminna, Rosaria (2004). "Oxidation of tartronic acid and dihydroxyacetone to sodium mesoxalate mediated by TEMPO". Tetrahedron Letters. 45 (34): 6381–6383. doi:10.1016/j.tetlet.2004.07.021.
- ^ Ciriminna, Rosaria (2003). "One-Pot Homogeneous and Heterogeneous Oxidation of Glycerol to Ketomalonic Acid Mediated by TEMPO". Advanced Synthesis & Catalysis. 345 (3): 383–388. doi:10.1002/adsc.200390043.