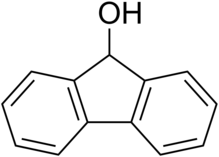
Fluorenol, also known as hydrafinil,[3] is an alcohol derivative of fluorene. In the most significant isomer, fluoren-9-ol or 9-hydroxyfluorene, the hydroxy group is located on the bridging carbon between the two benzene rings. Hydroxyfluorene can be converted to fluorenone by oxidation. It is a white-cream colored solid at room temperature.
| |

| |
| Names | |
|---|---|
| IUPAC name
9H-Fluoren-9-ol
| |
| Other names
9-Hydroxyfluorene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.015.345 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H10O | |
| Molar mass | 182.22 g/mol |
| Appearance | Off-white crystalline powder |
| Density | 1.151 g/mL |
| Melting point | 152 to 155 °C (306 to 311 °F; 425 to 428 K) |
| Practically insoluble [2] | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Toxicity
editFluorenol is toxic to aquatic organisms including algae, bacteria, and crustaceans.[4] Fluorenol was patented as an insecticide in 1939,[5] and is an algaecide against the green algae Dunaliella bioculata.[6]
Its toxicity and carcinogenicity in humans are unknown.[6]
Mechanism of action
editThe mechanism of action of fluorenol is unknown.[7]
The lipophilicity of fluorenol (LogP 2.4) is higher than that of drugs like modafinil (LogP 1.7) and amphetamine (LogP 1.8), suggesting that it may penetrate the blood brain barrier more readily.[8][9][10]
Eugeroic
editA study published by Cephalon describing research to develop a successor to the eugeroic modafinil reported that the corresponding fluorenol derivative was 39% more effective than modafinil at keeping mice awake over a 4-hour period.[11] However, after further investigation it was determined that the eugeroic activity of the fluorenol analog was likely due to an active metabolite, which they identify as fluorenol itself.[11] Fluorenol is a weak dopamine reuptake inhibitor with an IC50 of 9 μM, notably 59% weaker than modafinil (IC50 = 3.70 μM),[11] potentially making it even less liable for addiction.[12] It also showed no affinity for cytochrome P450 2C19, unlike modafinil.[11]
There is no evidence (binding assays, occupancy, predicted structure) to suggest that fluorenol acts as a 5-HT6 antagonist, contrary to some popular claims.[medical citation needed]
Sale as research chemical
editThe unscheduled nature of fluorenol has caused it to fall into a legal grey area in most countries. Despite being associated with modafinil,[13] fluorenol is not a substituted derivative of it, making its scheduling unimplied by analogue acts.
Fluorenol is a relatively obscure compound in the research chemical market. According to an online survey with over 3000 respondents, only 2% of modafinil users have reported using fluorenol.[14]
See also
editReferences
edit- ^ 9-Hydroxyfluorene, chemicalland21.com
- ^ Record of 9H-Fluoren-9-ol in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 5 November 2008.
- ^ Knoop, Andre; Fußhöller, Gregor; Haenelt, Nadine; Goergens, Christian; Guddat, Sven; Geyer, Hans; Thevis, Mario (November 2021). "Mass spectrometric characterization of urinary hydrafinil metabolites for routine doping control purposes". Drug Testing and Analysis. 13 (11–12): 1915–1920. doi:10.1002/dta.3137. ISSN 1942-7611. PMID 34378339. S2CID 236976954.
- ^ Šepič, Ester; Bricelj, Mihael; Leskovšek, Hermina (2003). "Toxicity of fluoranthene and its biodegradation metabolites to aquatic organisms". Chemosphere. 52 (7): 1125–33. Bibcode:2003Chmsp..52.1125S. doi:10.1016/S0045-6535(03)00321-7. PMID 12820993.
- ^ US patent 2197249: Insecticide
- ^ a b MSDS Archived 2016-03-04 at the Wayback Machine
- ^ Clifford W Fong. Modafinil and modafinil analogues: free radical mechanism of the eugeroic and cognitive enhancement effect. [Research Report] Eigenenergy. 2018. ffhal-01933737f
- ^ PubChem. "9H-Fluoren-9-ol". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-09-20.
- ^ PubChem. "Modafinil". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-09-20.
- ^ PubChem. "Amphetamine". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-09-20.
- ^ a b c d Dunn, D.; Hostetler, G.; Iqbal, M.; Marcy, V. R.; Lin, Y. G.; Jones, B.; Aimone, L. D.; Gruner, J.; Ator, M. A.; Bacon, E. R.; Chatterjee, S. (2012). "Wake promoting agents: Search for next generation modafinil, lessons learned: Part III". Bioorganic & Medicinal Chemistry Letters. 22 (11): 3751–3753. doi:10.1016/j.bmcl.2012.04.031. PMID 22546675.
- ^ Wise, R. A. (1996). "Neurobiology of addiction". Current Opinion in Neurobiology. 6 (2): 243–51. doi:10.1016/S0959-4388(96)80079-1. PMID 8725967. S2CID 25378856.
- ^ Dunn, Derek; Hostetler, Greg; Iqbal, Mohamed; Marcy, Val R.; Lin, Yin Guo; Jones, Bruce; Aimone, Lisa D.; Gruner, John; Ator, Mark A.; Bacon, Edward R.; Chatterjee, Sankar (2012-06-01). "Wake promoting agents: search for next generation modafinil, lessons learned: part III". Bioorganic & Medicinal Chemistry Letters. 22 (11): 3751–3753. doi:10.1016/j.bmcl.2012.04.031. ISSN 1464-3405. PMID 22546675.
- ^ Branwen, Gwern (2015-06-01). "Modafinil community survey".
{{cite journal}}: Cite journal requires|journal=(help)
