Glycogen synthase kinase 3 (GSK-3) is a serine/threonine protein kinase that mediates the addition of phosphate molecules onto serine and threonine amino acid residues. First discovered in 1980 as a regulatory kinase for its namesake, glycogen synthase (GS),[2] GSK-3 has since been identified as a protein kinase for over 100 different proteins in a variety of different pathways.[3][4] In mammals, including humans, GSK-3 exists in two isozymes encoded by two homologous genes GSK-3α (GSK3A) and GSK-3β (GSK3B). GSK-3 has been the subject of much research since it has been implicated in a number of diseases, including type 2 diabetes, Alzheimer's disease, inflammation, cancer, addiction[5] and bipolar disorder.
| Glycogen synthase kinase 3, catalytic domain | |
|---|---|
| Identifiers | |
| Symbol | STKc_GSK3 |
| InterPro | IPR039192 |
| CDD | cd14137 |
| glycogen synthase kinase 3 alpha | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | GSK3A | ||||||
| NCBI gene | 2931 | ||||||
| HGNC | 4616 | ||||||
| OMIM | 606784 | ||||||
| RefSeq | NM_019884 | ||||||
| UniProt | P49840 | ||||||
| Other data | |||||||
| EC number | 2.7.11.26 | ||||||
| Locus | Chr. 19 q13.2 | ||||||
| |||||||
| glycogen synthase kinase 3 beta | |||||||
|---|---|---|---|---|---|---|---|
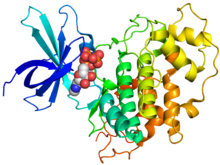 Crystallographic structure of human GSK-3β (rainbow colored, N-terminus = blue, C-terminus = red) bound to phosphoaminophosphonic acid-adenylate ester (spheres).[1] | |||||||
| Identifiers | |||||||
| Symbol | GSK3B | ||||||
| NCBI gene | 2932 | ||||||
| HGNC | 4617 | ||||||
| OMIM | 605004 | ||||||
| PDB | 1Q3W More structures | ||||||
| RefSeq | NM_002093 | ||||||
| UniProt | P49841 | ||||||
| Other data | |||||||
| EC number | 2.7.11.26 | ||||||
| Locus | Chr. 3 q13.33 | ||||||
| |||||||
GSK-3 is a serine/threonine protein kinase that phosphorylate either threonine or serine, and this phosphorylation controls a variety of biological activities, such as glycogen metabolism, cell signaling, cellular transport, and others.[6] GS inhibition by GSK-3β leads to a decrease in glycogen synthesis in the liver and muscles, along with increased blood glucose or hyperglycemia.[7] This is why GSK-3β is associated with the pathogenesis and progression of many diseases, such as diabetes, obesity, cancer,[8] and Alzheimer's disease.[9] It is active in resting cells and is inhibited by several hormones such as insulin, endothelial growth factor, and platelet-derived growth factor. Insulin indirectly inactivates GSK3 via downstream phosphorylation of the specific serine residues Ser21 and Ser9 in GSK-3 isoforms α and β, respectively via the PI3K/Akt pathway.[10][11]
As of 2019[update], GSK-3 is the only type of glycogen synthase kinase named and recognized. The gene symbols for GSK1 and GSK2 have been withdrawn by the HUGO Gene Nomenclature Committee (HGNC), and no new names for these "genes" nor their locations have been specified.[12][13]
Mechanism
editGSK-3 functions by phosphorylating a serine or threonine residue on its target substrate. A positively charged pocket adjacent to the active site binds a "priming" phosphate group attached to a serine or threonine four residues C-terminal of the target phosphorylation site. The active site, at residues 181, 200, 97, and 85, binds the terminal phosphate of ATP and transfers it to the target location on the substrate (see figure 1).[14]
Glycogen synthase
editGlycogen synthase is an enzyme that is responsible in glycogen synthesis. It is activated by glucose 6-phosphate (G6P), and inhibited by glycogen synthase kinases (GSK3). Those two mechanisms play an important role in glycogen metabolism.[15]
Function
editPhosphorylation of a protein by GSK-3 usually inhibits the activity of its downstream target.[16][17][18] GSK-3 is active in a number of central intracellular signaling pathways, including cellular proliferation, migration, glucose regulation, and apoptosis.
GSK-3 was originally discovered in the context of its involvement in regulating glycogen synthase.[2] After being primed by casein kinase 2 (CK2), glycogen synthase gets phosphorylated at a cluster of three C-terminal serine residues, reducing its activity.[19] In addition to its role in regulating glycogen synthase, GSK-3 has been implicated in other aspects of glucose homeostasis, including the phosphorylation of insulin receptor IRS1[20] and of the gluconeogenic enzymes phosphoenolpyruvate carboxykinase and glucose 6 phosphatase.[21] However, these interactions have not been confirmed, as these pathways can be inhibited without the up-regulation of GSK-3.[19]
GSK-3 has also been shown to regulate immune and migratory processes. GSK-3 participates in a number of signaling pathways in the innate immune response, including pro-inflammatory cytokine and interleukin production.[22][23] The inactivation of GSK3B by various protein kinases also affects the adaptive immune response by inducing cytokine production and proliferation in naïve and memory CD4+ T cells.[23] In cellular migration, an integral aspect of inflammatory responses, the inhibition of GSK-3 has been reported to play conflicting roles, as local inhibition at growth cones has been shown to promote motility while global inhibition of cellular GSK-3 has been shown to inhibit cell spreading and migration.[22]
GSK-3 is also integrally tied to pathways of cell proliferation and apoptosis. GSK-3 has been shown to phosphorylate Beta-catenin, thus targeting it for degradation.[24] GSK-3 is therefore a part of the canonical Beta-catenin/Wnt pathway, which signals the cell to divide and proliferate. GSK-3 phosphorylates cyclins D and E, which are important for the transition from G1 to S phase, and causes their degradation. The transcription factors c-myc and c-fos (also S phase promoters ), which are primarily phosphorylated by the dual-specificity tyrosine phosphorylation-regulated kinase, are also phosphorylated by GSK3, causing them to be degraded.[25] GSK-3 also participates in a number of apoptotic signaling pathways by phosphorylating transcription factors that regulate apoptosis.[4] GSK-3 can promote apoptosis by both activating pro-apoptotic factors such as p53[26] and inactivating survival-promoting factors through phosphorylation.[27] The role of GSK-3 in regulating apoptosis is controversial, however, as some studies have shown that GSK-3β knockout mice are overly sensitized to apoptosis and die in the embryonic stage, while others have shown that overexpression of GSK-3 can induce apoptosis.[28] Overall, GSK-3 appears to both promote and inhibit apoptosis, and this regulation varies depending on the specific molecular and cellular context.[29]
GSK-3 is also involved in nuclear transcriptional activator kappa B (NFκB) signaling pathway, Hedgehog signaling pathway, Notch signaling pathway, and epithelial-mesenchymal transition.[25]
Due to its importance across numerous cellular functions, GSK-3 activity is subject to tight regulation and is considered an "Ace" among kinases.[30]
The speed and efficacy of GSK-3 phosphorylation is regulated by several factors. Phosphorylation of certain GSK-3 residues can increase or decrease its ability to bind substrate. Phosphorylation at tyrosine-216 in GSK-3β or tyrosine-279 in GSK-3α enhances the enzymatic activity of GSK-3, while phosphorylation of autoinhibitory serine-9 in GSK-3β or serine-21 in GSK-3α significantly decreases active site availability (see figure).[22] Further, GSK-3 is unusual among kinases in that it usually requires a "priming kinase" to first phosphorylate a substrate. A phosphorylated serine or threonine residue located four amino acids C-terminal to the target site of phosphorylation allows the substrate to bind a pocket of positive charge formed by arginine and lysine residues.[19][31]
Depending on the pathway in which it is being utilized, GSK-3 may be further regulated by cellular localization or the formation of protein complexes. The activity of GSK-3 is far greater in the nucleus and mitochondria than in the cytosol in cortical neurons,[32] while the phosphorylation of Beta-catenin by GSK-3 is mediated by the binding of both proteins to Axin, a scaffold protein, allowing Beta-catenin to access the active site of GSK-3.[22]
Insulin indirectly inactivates GSK3 via downstream phosphorylation of the specific serine residues Ser21 and Ser9 in GSK-3 isoforms α and β, respectively, via the PI3K/Akt pathway (protein kinase B).[10][11]
Disease relevance
editDue to its involvement in a great number of signaling pathways, GSK-3 has been associated with a host of high-profile diseases. GSK-3 inhibitors are currently being tested for therapeutic effects in Alzheimer's disease, type 2 diabetes mellitus (T2DM), some forms of cancer, and bipolar disorder.[33]
There is evidence that lithium, which is used as a treatment for bipolar disorder, acts as a mood stabilizer by selectively inhibiting GSK-3. The mechanism through which GSK-3 inhibition may stabilize mood is not known, though it is suspected that the inhibition of GSK-3's ability to promote inflammation contributes to the therapeutic effect.[22] Inhibition of GSK-3 also destabilises Rev-ErbA alpha transcriptional repressor, which has a significant role in the circadian clock.[34] Elements of the circadian clock may be connected with predisposition to bipolar mood disorder.[35]
GSK-3 activity has been associated with both pathological features of Alzheimer's disease, namely the buildup of amyloid-β (Aβ) deposits and the formation of neurofibrillary tangles. GSK-3 is thought to directly promote Aβ production and to be tied to the process of the hyperphosphorylation of tau proteins, which leads to the tangles.[4][22] Due to these roles of GSK-3 in promoting Alzheimer's disease, GSK-3 inhibitors may have positive therapeutic effects on Alzheimer's patients and are currently in the early stages of testing.[36]
In a similar fashion, targeted inhibition of GSK-3 may have therapeutic effects on certain kinds of cancer. Though GSK-3 has been shown to promote apoptosis in some cases, it has also been reported to be a key factor in tumorigenesis in some cancers.[37] Supporting this claim, GSK-3 inhibitors have been shown to induce apoptosis in glioma and pancreatic cancer cells.[28][38] GSK-3 also seems to be responsible for NFκB aberrant activity in pediatric acute lymphoblastic leukemia and pancreatic cancer cells. In renal cancer cells, GSK-3 inhibitors induce cell cycle arrest, differentiation of the malignant cells, and autophagy. In contrast to the above neoplasms, high expression of inactive pGSK3β-S9 is found in skin, oral, and lung cancers, suggesting tumor suppressive effects of the enzyme in these cancers. In melanoma, the microRNA miR-769 inhibits GSK-3 activity during the tumor development process, also indicating tumor suppressive effects of GSK3.[25]
GSK-3 inhibitors have also shown promise in the treatment of T2DM.[19] Though GSK-3 activity under diabetic conditions can differ radically across different tissue types, studies have shown that introducing competitive inhibitors of GSK-3 can increase glucose tolerance in diabetic mice.[22] GSK-3 inhibitors may also have therapeutic effects on hemorrhagic transformation after acute ischemic stroke.[39] GSK-3 can negatively regulate the insulin signaling pathway by inhibiting IRS1 via phosphorylation of serine-332,[20] rendering the insulin receptor incapable of activating IRS1 and further initiating the canonical PI3K/Akt pathway. The role that inhibition of GSK-3 might play across its other signaling roles is not yet entirely understood.
GSK-3 inhibition also mediates an increase in the transcription of the transcription factor Tbet (Tbx21) and an inhibition of the transcription of the inhibitory co-receptor programmed cell death-1 (PD-1) on T-cells.[40] GSK-3 inhibitors increased in vivo CD8(+) OT-I CTL function and the clearance of viral infections by murine gamma-herpesvirus 68 and lymphocytic choriomeningitis clone 13 as well as anti-PD-1 in immunotherapy.
Inhibitors
editGlycogen synthase kinase inhibitors are different chemotypes and have variable mechanisms of action; they may be cations, from natural sources, synthetic ATP and non-ATP competitive inhibitors and substrate-competitive inhibitors. GSK3 is a bi-lobar architecture with N-terminal and C-terminal, the N-terminal is responsible for ATP binding and C-terminal which is called as activation loop mediates the kinase activity, Tyrosine located at the C-terminal it essential for full GSK3 activity.[41]
Benefits of GSK-3β inhibitors
editIn diabetes, GSK-3β inhibitors increase insulin sensitivity, glycogen synthesis, and glucose metabolism in skeletal muscles, and reduce obesity by affecting the adipogenesis process.[42] GSK-3β is also over expressed in several types of cancers, like colorectal, ovarian, and prostate cancer.[41] GSK-3β inhibitors also aid in the treatment of Alzheimer's disease,[citation needed] stroke,[39] and mood disorders, including bipolar disorder.[43] In vitro studies have shown the beneficial effects of GSK-3 inhibitors in lung cancer,[44] ovarian cancer[45] and neuroblastoma.[46]
Specific agents
editInhibitors of GSK-3 include:[47][48][49][50]
Metal cations
editATP-competitive
editMarine organism-derived
edit- 6-BIO (IC50=1.5μM)
- Dibromocantharelline (IC50=3μM)
- Hymenialdesine (IC50=10nM)
- Indirubin (IC50=5-50nM)
- Meridianin
Aminopyrimidines
editArylindolemaleimide
editThiazoles
edit- AR-A014418 (IC50=104nM)
- AZD-1080 (IC50=6.9nM-31nM)
Paullones
editIC50=4-80nM:
Aloisines
editIC50=0.5-1.5μM:
Non-ATP competitive
editMarine organism-derived
edit- Manzamine A (IC50=1.5μM)
- Palinurine (IC50=4.5μM)
- Tricantine (IC50=7.5μM)
Thiazolidinediones
edit- TDZD-8 (IC50=2μM)
- NP00111 (IC50=2μM)
- NP031115 (IC50=4μM)
- Tideglusib (IC50=60nM)
Halomethylketones
edit- HMK-32 (IC50=1.5μM)
Peptides
editUnknown Mechanism (small-molecule inhibitors)
editLithium
editLithium which is used in the treatment of bipolar disorder was the first natural GSK-3 inhibitor discovered. It inhibits GSK-3 directly by competition with magnesium ions and indirectly by phosphorylation and auto-regulation of serine. Lithium has been found to have insulin-like effects on glucose metabolism, including stimulation of glycogen synthesis in fat cells, skin, and muscles, increasing glucose uptake, and activation of GS activity. In addition to inhibition of GSK-3, it also inhibits other enzymes involved in the regulation of glucose metabolisms, such as myo-inositol-1-monophosphatase and 1,6 bisphosphatase. Also, it has shown therapeutic benefit in Alzheimer's and other neurodegenerative diseases such as epileptic neurodegeneration.[49]
Naproxen and Cromolyn
editNaproxen is a non-steroidal anti-inflammatory drug while cromolyn is an anti-allergic agent which acts as a mast cell stabilizer. Both drugs have demonstrated the anticancer effect in addition to hypoglycemic effect due to inhibition of glycogen synthase kinase-3β (GSK-3β).
To validate the anti-GSK-3β hypothesis of naproxen and cromolyn, docking of the two structures against GSK-3β binding pocket and comparing their fitting with known GSK-3β inhibitor ARA014418 was performed, in addition to measuring the serum glucose, serum insulin, serum C-peptide, weight variation and hepatic glycogen levels for normal and diabetic fasting animal's models to assess their in vitro hypoglycemic effects.[citation needed]
Naproxen and cromolyn were successfully docked into the binding site of GSK-3β (both were fitted into its binding pocket). They exhibited electrostatic, hydrophobic, and hydrogen-bonding interactions with key amino acids within the binding pocket with binding interaction profiles similar to AR-A014418 (the known inhibitor). The negative charges of the carboxylic acid groups in both drugs interact electrostatically with the positively charged guanidine group of Arg141. Moreover, the hydrogen bonding interactions between carboxylic acid moieties of cromolyn and the ammonium groups of Lys183 and Lys60, in addition to π-stacking of the naphthalene ring system of naproxen with the phenolic ring of Tyr134.
Antidiabetic effects of naproxen and cromolyn: In normal animal models, both drugs have showed dose-dependent reduction in blood glucose levels and rise in glycogen levels. In chronic type II diabetic model, glucose levels were also reduced, and glycogen level and insulin levels were elevated in a dose-dependent manner with a reduction in plasma glucose.[citation needed]
Anti-obesity effects of naproxen and cromolyn: Both drugs showed significant anti-obesity effects as they reduce body weight, resistin, and glucose levels in a dose-dependent manner. They were also found to elevate adiponectin, insulin, and C-peptide levels in a dose-dependent manner.[42]
Famotidine
editFamotidine is a specific, long-acting H2 antagonist that decreases gastric acid secretion. It is used in the treatment of peptic ulcer disease, GERD, and pathological hypersecretory conditions, like Zollinger–Ellison syndrome. (14,15) H2-receptor antagonists affect hormone metabolism, but their effect on glucose metabolism is not well established. (16) A study has revealed a glucose-lowering effect for famotidine.[citation needed]
The study of famotidine binding to the enzyme has showed that famotidine can be docked within the binding pocket of GSK-3β making significant interactions with key points within the GSK-3β binding pocket. Strong hydrogen bond interactions with the key amino acids PRO-136 and VAL -135 and potential hydrophobic interaction with LEU-188 were similar to those found in the ligand binding to the enzyme (AR-A014418).[citation needed]
Furthermore, famotidine showed high GSK-3β binding affinity and inhibitory activity due to interactions that stabilize the complex, namely hydrogen bonding of guanidine group in famotidine with the sulfahydryl moiety in CYS-199; and electrostatic interactions between the same guanidine group with the carboxyl group in ASP-200, the hydrogen bond between the terminal NH2 group, the OH of the TYR-143, and the hydrophobic interaction of the sulfur atom in the thioether with ILE-62. In vitro studies showed that famotidine inhibits GSK-3β activity and increases liver glycogen reserves in a dose dependent manner. A fourfold increase in the liver glycogen level with the use of the highest dose of famotidine (4.4 mg/kg) was observed. Also, famotidine has been shown to decrease serum glucose levels 30, and 60 minutes after oral glucose load in healthy individuals.[51]
Curcumin
editCurcumin, which Is a constituent of turmeric spice, has flavoring and coloring properties.[52] It has two symmetrical forms: enol (the most abundant forms) and ketone.[53][54]
Curcumin has wide pharmacological activities: anti-inflammatory,[55] anti-microbial,[56] hypoglycemic, anti-oxidant, and wound healing effects.[57] In animal models with Alzheimer disease, it has anti-destructive effect of beta amyloid in the brain,[58] and recently it shows anti-malarial activity.[59]
Curcumin also has chemo preventative and anti-cancer effects,[citation needed] and it has been shown to attenuate oxidative stress and renal dysfunction in diabetic animals with chronic use.[60]
Curcumin's mechanism of action is anti-inflammatory; it inhibits the nuclear transcriptional activator kappa B (NF-KB) that is activated whenever there is inflammatory response.[citation needed]
NF-kB has two regulatory factors, IkB and GSK-3,[61] which suggests curcumin directly binds and inhibits GSK-3B. An in vitro study confirmed GSK-3B inhibition by simulating molecular docking using a silico docking technique.[62] The concentration at which 50% of GK-3B would be inhibited by curcumin is 66.3 nM.[62]
Among its two forms, experimental and theoretical studies show that the enol form is the favored form due to its intra-molecular hydrogen bonding, and an NMR experiment show that enol form exist in a variety of solvents.[citation needed]
Olanzapine
editAntipsychotic medications are increasingly used for schizophrenia, bipolar disorder, anxiety, and other psychiatric conditions[63] Atypical antipsychotics are more commonly used than first generation antipsychotics because they decrease the risk of extrapyramidal symptoms, such as tardive dyskinesia, and have better efficacy.[64]
Olanzapine and atypical antipsychotics induce weight gain through increasing body fat.[65] It also affects glucose metabolism, and several studies shows that it may worsen diabetes.[66]
A recent study shows that olanzapine inhibits GSK3 activity, suggesting olanzapine permits glycogen synthesis. A study of the effect of olanzapine on mouse blood glucose and glycogen levels showed a significant decrease in blood glucose level and elevation of glycogen level in mice, and the IC50% of olanzapine were 91.0 nm, which is considered a potent inhibitor. The study also illustrates that sub-chronic use of olanzapine results in potent inhibition of GSK3.[43]
Pyrimidine derivatives
editPyrimidine analogues are antimetabolites that interfere with nucleic acid synthesis.[67] Some of them have been shown to fit the ATP-binding pocket of GSK-3β to lower blood glucose levels and improve some neuronal diseases.[68]
See also
editReferences
edit- ^ PDB: 1J1B; Aoki M, Yokota T, Sugiura I, Sasaki C, Hasegawa T, Okumura C, et al. (March 2004). "Structural insight into nucleotide recognition in tau-protein kinase I/glycogen synthase kinase 3 beta". Acta Crystallographica. Section D, Biological Crystallography. 60 (Pt 3): 439–446. Bibcode:2004AcCrD..60..439A. doi:10.1107/S090744490302938X. PMID 14993667.
- ^ a b Embi N, Rylatt DB, Cohen P (June 1980). "Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase". European Journal of Biochemistry. 107 (2): 519–527. doi:10.1111/j.1432-1033.1980.tb06059.x. PMID 6249596.
- ^ Beurel E, Grieco SF, Jope RS (April 2015). "Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases". Pharmacology & Therapeutics. 148: 114–131. doi:10.1016/j.pharmthera.2014.11.016. PMC 4340754. PMID 25435019.
- ^ a b c Jope RS, Johnson GV (February 2004). "The glamour and gloom of glycogen synthase kinase-3". Trends in Biochemical Sciences. 29 (2): 95–102. doi:10.1016/j.tibs.2003.12.004. PMID 15102436.
- ^ Turlik J, Wąsikiewicz E, Domaradzka A, Chrostek G, Gniadzik W, Domagalski M, Duda P (December 2021). "GSK3β Activity in Reward Circuit Functioning and Addiction". NeuroSci. 2 (4): 443–466. doi:10.3390/neurosci2040033. ISSN 2673-4087.
- ^ Pandey MK, DeGrado TR (2016). "Glycogen Synthase Kinase-3 (GSK-3)-Targeted Therapy and Imaging". Theranostics. 6 (4): 571–593. doi:10.7150/thno.14334. PMC 4775866. PMID 26941849.
- ^ Ali A, Hoeflich KP, Woodgett JR (August 2001). "Glycogen synthase kinase-3: properties, functions, and regulation". Chemical Reviews. 101 (8): 2527–2540. doi:10.1021/cr000110o. PMID 11749387.
- ^ Eldar-Finkelman H (March 2002). "Glycogen synthase kinase 3: an emerging therapeutic target". Trends in Molecular Medicine. 8 (3): 126–132. doi:10.1016/S1471-4914(01)02266-3. PMID 11879773.
- ^ Hooper C, Killick R, Lovestone S (March 2008). "The GSK3 hypothesis of Alzheimer's disease". Journal of Neurochemistry. 104 (6): 1433–1439. doi:10.1111/j.1471-4159.2007.05194.x. PMC 3073119. PMID 18088381.
- ^ a b Hermida MA, Kumar JD, Leslie NR (August 2017). "GSK3 and its interactions with the PI3K/AKT/mTOR signalling network". Advances in Biological Regulation. 65: 5–15. doi:10.1016/j.jbior.2017.06.003. PMID 28712664. Retrieved 15 December 2023.
- ^ a b Li Q, Zhao Q, Zhang J, Linkang L, Wenhao W, Chua B, Chen Y, Xu L, Li P (September 24, 2019). "The Protein Phosphatase 1 Complex Is a Direct Target of AKT that Links Insulin Signaling to Hepatic Glycogen Deposition". Cell Reports. 28 (13): 3406–3422. doi:10.1016/j.celrep.2019.08.066. PMID 31553910.
- ^ Glycogen+synthase+kinase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ^ GSK1, GSK2. NCBI Gene.
- ^ Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, Pearl LH (June 2001). "Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition". Cell. 105 (6): 721–732. doi:10.1016/S0092-8674(01)00374-9. PMID 11440715. S2CID 17401752.
- ^ Bouskila M, Hunter RW, Ibrahim AF, Delattre L, Peggie M, van Diepen JA, et al. (November 2010). "Allosteric regulation of glycogen synthase controls glycogen synthesis in muscle". Cell Metabolism. 12 (5): 456–466. doi:10.1016/j.cmet.2010.10.006. PMID 21035757.
- ^ Woodgett JR (August 1994). "Regulation and functions of the glycogen synthase kinase-3 subfamily". Seminars in Cancer Biology. 5 (4): 269–275. PMID 7803763.
- ^ Woodgett JR (September 2001). "Judging a protein by more than its name: GSK-3". Science's STKE. 2001 (100): re12. doi:10.1126/stke.2001.100.re12. PMID 11579232. S2CID 19052833.
- ^ Ali A, Hoeflich KP, Woodgett JR (August 2001). "Glycogen synthase kinase-3: properties, functions, and regulation". Chemical Reviews. 101 (8): 2527–2540. doi:10.1021/cr000110o. PMID 11749387. S2CID 12925005.
- ^ a b c d Rayasam GV, Tulasi VK, Sodhi R, Davis JA, Ray A (March 2009). "Glycogen synthase kinase 3: more than a namesake". British Journal of Pharmacology. 156 (6): 885–898. doi:10.1111/j.1476-5381.2008.00085.x. PMC 2697722. PMID 19366350.
- ^ a b Liberman Z, Eldar-Finkelman H (February 2005). "Serine 332 phosphorylation of insulin receptor substrate-1 by glycogen synthase kinase-3 attenuates insulin signaling". The Journal of Biological Chemistry. 280 (6): 4422–4428. doi:10.1074/jbc.M410610200. PMID 15574412.
- ^ Lochhead PA, Coghlan M, Rice SQ, Sutherland C (May 2001). "Inhibition of GSK-3 selectively reduces glucose-6-phosphatase and phosphatase and phosphoenolypyruvate carboxykinase gene expression". Diabetes. 50 (5): 937–946. doi:10.2337/diabetes.50.5.937. PMID 11334436.
- ^ a b c d e f g Jope RS, Yuskaitis CJ, Beurel E (Apr–May 2007). "Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics". Neurochemical Research. 32 (4–5): 577–595. doi:10.1007/s11064-006-9128-5. PMC 1970866. PMID 16944320.
- ^ a b Wang H, Brown J, Martin M (February 2011). "Glycogen synthase kinase 3: a point of convergence for the host inflammatory response". Cytokine. 53 (2): 130–140. doi:10.1016/j.cyto.2010.10.009. PMC 3021641. PMID 21095632.
- ^ Mills CN, Nowsheen S, Bonner JA, Yang ES (2011). "Emerging roles of glycogen synthase kinase 3 in the treatment of brain tumors". Frontiers in Molecular Neuroscience. 4: 47. doi:10.3389/fnmol.2011.00047. PMC 3223722. PMID 22275880.
- ^ a b c Glibo M, Serman A, Karin-Kujundzic V, Bekavac Vlatkovic I, Miskovic B, Vranic S, Serman L (February 2021). "The role of glycogen synthase kinase 3 (GSK3) in cancer with emphasis on ovarian cancer development and progression: A comprehensive review". Bosnian Journal of Basic Medical Sciences. 21 (1): 5–18. doi:10.17305/bjbms.2020.5036. PMC 7861620. PMID 32767962.
- ^ Watcharasit P, Bijur GN, Zmijewski JW, Song L, Zmijewska A, Chen X, et al. (June 2002). "Direct, activating interaction between glycogen synthase kinase-3beta and p53 after DNA damage". Proceedings of the National Academy of Sciences of the United States of America. 99 (12): 7951–7955. Bibcode:2002PNAS...99.7951W. doi:10.1073/pnas.122062299. PMC 123001. PMID 12048243.
- ^ Grimes CA, Jope RS (September 2001). "CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium". Journal of Neurochemistry. 78 (6): 1219–1232. doi:10.1046/j.1471-4159.2001.00495.x. PMC 1947002. PMID 11579131.
- ^ a b Kotliarova S, Pastorino S, Kovell LC, Kotliarov Y, Song H, Zhang W, et al. (August 2008). "Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-kappaB, and glucose regulation". Cancer Research. 68 (16): 6643–6651. doi:10.1158/0008-5472.CAN-08-0850. PMC 2585745. PMID 18701488.
- ^ Jacobs KM, Bhave SR, Ferraro DJ, Jaboin JJ, Hallahan DE, Thotala D (May 2012). "GSK-3β: A Bifunctional Role in Cell Death Pathways". International Journal of Cell Biology. 2012: 930710. doi:10.1155/2012/930710. PMC 3364548. PMID 22675363.
- ^ Mathuram TL (15 May 2024). "GSK-3: An "Ace" Among Kinases". Cancer biotherapy & radiopharmaceuticals. doi:10.1089/cbr.2024.0025. PMID 38746994.
- ^ Doble BW, Woodgett JR (April 2003). "GSK-3: tricks of the trade for a multi-tasking kinase". Journal of Cell Science. 116 (Pt 7): 1175–1186. doi:10.1242/jcs.00384. PMC 3006448. PMID 12615961.
- ^ Bijur GN, Jope RS (December 2003). "Glycogen synthase kinase-3 beta is highly activated in nuclei and mitochondria". NeuroReport. 14 (18): 2415–2419. doi:10.1097/00001756-200312190-00025. PMID 14663202. S2CID 43633965.
- ^ Saraswati AP, Ali Hussaini SM, Krishna NH, Babu BN, Kamal A (January 2018). "Glycogen synthase kinase-3 and its inhibitors: Potential target for various therapeutic conditions". European Journal of Medicinal Chemistry. 144: 843–858. doi:10.1016/j.ejmech.2017.11.103. PMID 29306837.
- ^ Yin L, Wang J, Klein PS, Lazar MA (February 2006). "Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock". Science. 311 (5763): 1002–1005. Bibcode:2006Sci...311.1002Y. doi:10.1126/science.1121613. PMID 16484495. S2CID 11240826.
- ^ Rybakowski JK, Dmitrzak-Weglarz M, Dembinska-Krajewska D, Hauser J, Akiskal KK, Akiskal HH (April 2014). "Polymorphism of circadian clock genes and temperamental dimensions of the TEMPS-A in bipolar disorder". Journal of Affective Disorders. 159: 80–84. doi:10.1016/j.jad.2014.02.024. PMID 24679394.
- ^ Hu S, Begum AN, Jones MR, Oh MS, Beech WK, Beech BH, et al. (February 2009). "GSK3 inhibitors show benefits in an Alzheimer's disease (AD) model of neurodegeneration but adverse effects in control animals". Neurobiology of Disease. 33 (2): 193–206. doi:10.1016/j.nbd.2008.10.007. PMC 4313761. PMID 19038340.
- ^ Wang Z, Smith KS, Murphy M, Piloto O, Somervaille TC, Cleary ML (October 2008). "Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy". Nature. 455 (7217): 1205–1209. Bibcode:2008Natur.455.1205W. doi:10.1038/nature07284. PMC 4084721. PMID 18806775.
- ^ Marchand B, Tremblay I, Cagnol S, Boucher MJ (March 2012). "Inhibition of glycogen synthase kinase-3 activity triggers an apoptotic response in pancreatic cancer cells through JNK-dependent mechanisms". Carcinogenesis. 33 (3): 529–537. doi:10.1093/carcin/bgr309. PMID 22201186.
- ^ a b Wang W, Li M, Wang Y, Li Q, Deng G, Wan J, et al. (December 2016). "GSK-3β inhibitor TWS119 attenuates rtPA-induced hemorrhagic transformation and activates the Wnt/β-catenin signaling pathway after acute ischemic stroke in rats". Molecular Neurobiology. 53 (10): 7028–7036. doi:10.1007/s12035-015-9607-2. PMC 4909586. PMID 26671619.
- ^ Taylor A, Harker JA, Chanthong K, Stevenson PG, Zuniga EI, Rudd CE (February 2016). "Glycogen Synthase Kinase 3 Inactivation Drives T-bet-Mediated Downregulation of Co-receptor PD-1 to Enhance CD8(+) Cytolytic T Cell Responses". Immunity. 44 (2): 274–286. doi:10.1016/j.immuni.2016.01.018. PMC 4760122. PMID 26885856.
- ^ a b Sayas CL, Ariaens A, Ponsioen B, Moolenaar WH (April 2006). "GSK-3 is activated by the tyrosine kinase Pyk2 during LPA1-mediated neurite retraction". Molecular Biology of the Cell. 17 (4): 1834–1844. doi:10.1091/mbc.E05-07-0688. PMC 1415316. PMID 16452634.
- ^ a b Motawi TM, Bustanji Y, El-Maraghy SA, Taha MO, Al Ghussein MA (September 2013). "Naproxen and cromolyn as new glycogen synthase kinase 3β inhibitors for amelioration of diabetes and obesity: an investigation by docking simulation and subsequent in vitro/in vivo biochemical evaluation". Journal of Biochemical and Molecular Toxicology. 27 (9): 425–436. doi:10.1002/jbt.21503. PMID 23784744. S2CID 46597394.
- ^ a b Mohammad MK, Al-Masri IM, Taha MO, Al-Ghussein MA, Alkhatib HS, Najjar S, Bustanji Y (April 2008). "Olanzapine inhibits glycogen synthase kinase-3beta: an investigation by docking simulation and experimental validation". European Journal of Pharmacology. 584 (1): 185–191. doi:10.1016/j.ejphar.2008.01.019. PMID 18295757.
- ^ Mathuram TL, Venkatesan T, Das J, Natarajan U, Rathinavelu A (August 2020). "The apoptotic effect of GSK-3 inhibitors: BIO and CHIR 98014 on H1975 lung cancer cells through ROS generation and mitochondrial dysfunction". Biotechnology Letters. 42 (8): 1351–1368. doi:10.1007/s10529-020-02861-w. ISSN 0141-5492.
- ^ Mathuram TL, Ravikumar V, Reece LM, Sasikumar CS, Cherian KM (2017). "Correlative Studies Unravelling the Possible Mechanism of Cell Death in Tideglusib-Treated Human Ovarian Teratocarcinoma-Derived PA-1 Cells". Journal of Environmental Pathology, Toxicology and Oncology. 36 (4): 321–344. doi:10.1615/JEnvironPatholToxicolOncol.2017025018. ISSN 0731-8898.
- ^ Mathuram TL, Ravikumar V, Reece LM, Karthik S, Sasikumar CS, Cherian KM (September 2016). "Tideglusib induces apoptosis in human neuroblastoma IMR32 cells, provoking sub-G 0 /G 1 accumulation and ROS generation". Environmental Toxicology and Pharmacology. 46: 194–205. doi:10.1016/j.etap.2016.07.013.
- ^ Noori MS, Bhatt PM, Courreges MC, Ghazanfari D, Cuckler C, Orac CM, et al. (December 2019). "Identification of a novel selective and potent inhibitor of glycogen synthase kinase-3". American Journal of Physiology. Cell Physiology. 317 (6): C1289–C1303. doi:10.1152/ajpcell.00061.2019. PMC 6962522. PMID 31553649.
- ^ Licht-Murava A, Paz R, Vaks L, Avrahami L, Plotkin B, Eisenstein M, Eldar-Finkelman H (November 2016). "A unique type of GSK-3 inhibitor brings new opportunities to the clinic". Science Signaling. 9 (454): ra110. doi:10.1126/scisignal.aah7102. PMID 27902447. S2CID 34207388.
- ^ a b Eldar-Finkelman H, Martinez A (2011). "GSK-3 Inhibitors: Preclinical and Clinical Focus on CNS". Frontiers in Molecular Neuroscience. 4: 32. doi:10.3389/fnmol.2011.00032. PMC 3204427. PMID 22065134.
- ^ McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Sokolosky M, Abrams SL, et al. (May 2014). "GSK-3 as potential target for therapeutic intervention in cancer". Oncotarget. 5 (10): 2881–2911. doi:10.18632/oncotarget.2037. PMC 4102778. PMID 24931005.
- ^ Mohammad M, Al-Masri IM, Issa A, Al-Ghussein MA, Fararjeh M, Alkhatib H, et al. (August 2013). "Famotidine inhibits glycogen synthase kinase-3β: an investigation by docking simulation and experimental validation". Journal of Enzyme Inhibition and Medicinal Chemistry. 28 (4): 690–694. doi:10.3109/14756366.2012.672413. PMID 22512725. S2CID 11890710.
- ^ Maheshwari RK, Singh AK, Gaddipati J, Srimal RC (March 2006). "Multiple biological activities of curcumin: a short review". Life Sciences. 78 (18): 2081–2087. doi:10.1016/j.lfs.2005.12.007. PMID 16413584.
- ^ Balasubramanian K (May 2006). "Molecular orbital basis for yellow curry spice curcumin's prevention of Alzheimer's disease". Journal of Agricultural and Food Chemistry. 54 (10): 3512–3520. doi:10.1021/jf0603533. PMID 19127718.
- ^ Payton F, Sandusky P, Alworth WL (February 2007). "NMR study of the solution structure of curcumin". Journal of Natural Products. 70 (2): 143–146. doi:10.1021/np060263s. PMID 17315954.
- ^ Kohli K, Ali J, Ansari MJ, Raheman Z (2005). "Curcumin: A natural antiinflammatory agent". Indian Journal of Pharmacology. 37 (3): 141. doi:10.4103/0253-7613.16209. hdl:1807/8668.
- ^ Negi PS, Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK (October 1999). "Antibacterial activity of turmeric oil: a byproduct from curcumin manufacture". Journal of Agricultural and Food Chemistry. 47 (10): 4297–4300. doi:10.1021/jf990308d. PMID 10552805.
- ^ Sidhu GS, Singh AK, Thaloor D, Banaudha KK, Patnaik GK, Srimal RC, Maheshwari RK (1998). "Enhancement of wound healing by curcumin in animals". Wound Repair and Regeneration. 6 (2): 167–177. doi:10.1046/j.1524-475X.1998.60211.x. PMID 9776860. S2CID 21440334.
- ^ Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, et al. (February 2005). "Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo". The Journal of Biological Chemistry. 280 (7): 5892–5901. doi:10.1074/jbc.M404751200. PMID 15590663.
- ^ Mishra S, Karmodiya K, Surolia N, Surolia A (March 2008). "Synthesis and exploration of novel curcumin analogues as anti-malarial agents". Bioorganic & Medicinal Chemistry. 16 (6): 2894–2902. doi:10.1016/j.bmc.2007.12.054. PMID 18194869.
- ^ Sharma S, Kulkarni SK, Chopra K (October 2006). "Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats". Clinical and Experimental Pharmacology & Physiology. 33 (10): 940–945. doi:10.1111/j.1440-1681.2006.04468.x. PMID 17002671. S2CID 25193929.
- ^ Demarchi F, Bertoli C, Sandy P, Schneider C (October 2003). "Glycogen synthase kinase-3 beta regulates NF-kappa B1/p105 stability". The Journal of Biological Chemistry. 278 (41): 39583–39590. doi:10.1074/jbc.M305676200. PMID 12871932.
- ^ a b Bustanji Y, Taha MO, Almasri IM, Al-Ghussein MA, Mohammad MK, Alkhatib HS (June 2009). "Inhibition of glycogen synthase kinase by curcumin: Investigation by simulated molecular docking and subsequent in vitro/in vivo evaluation". Journal of Enzyme Inhibition and Medicinal Chemistry. 24 (3): 771–778. doi:10.1080/14756360802364377. PMID 18720192. S2CID 23137441.
- ^ "Antipsychotics A-Z". Mind.org.uk. 2018.[unreliable medical source?]
- ^ "Antipsychotic Medication for Bipolar Disorder". WebMD.
- ^ Goudie AJ, Smith JA, Halford JC (December 2002). "Characterization of olanzapine-induced weight gain in rats". Journal of Psychopharmacology. 16 (4): 291–296. doi:10.1177/026988110201600402. PMID 12503827. S2CID 23589812.
- ^ Di Lorenzo R, Brogli A (September 2010). "Profile of olanzapine long-acting injection for the maintenance treatment of adult patients with schizophrenia". Neuropsychiatric Disease and Treatment. 6: 573–581. doi:10.2147/NDT.S5463. PMC 2938306. PMID 20856920.
- ^ Murphy F, Middleton M (2012). "Cytostatic and cytotoxic drugs". A worldwide yearly survey of new data in adverse drug reactions and interactions. Side Effects of Drugs Annual. Vol. 34. pp. 731–747. doi:10.1016/B978-0-444-59499-0.00045-3. ISBN 978-0-444-59499-0.
- ^ Kramer T, Schmidt B, Lo Monte F (2012). "Small-Molecule Inhibitors of GSK-3: Structural Insights and Their Application to Alzheimer's Disease Models". International Journal of Alzheimer's Disease. 2012: 381029. doi:10.1155/2012/381029. PMC 3408674. PMID 22888461.
External links
edit- Glycogen Synthase Kinase 3 at the U.S. National Library of Medicine Medical Subject Headings (MeSH)