Iron(II) bromide refers to inorganic compounds with the chemical formula FeBr2(H2O)x. The anhydrous compound (x = 0) is a yellow or brownish-colored paramagnetic solid. The tetrahydrate is also known, all being pale colored solids. They are common precursor to other iron compounds.

| |
| |
| Names | |
|---|---|
| IUPAC name
Iron(II) bromide
| |
| Other names
Ferrous bromide
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.029.244 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| FeBr2 | |
| Molar mass | 215.65 g mol−1 |
| Appearance | yellow-brown solid |
| Density | 4.63 g cm−3, solid |
| Melting point | 684 °C (1,263 °F; 957 K) (anhydrous) 27 °C (Hexahydrate) |
| Boiling point | 934 °C (1,713 °F; 1,207 K) |
| 117 g / 100 ml | |
| Solubility in other solvents | THF, methanol, ethanol |
| +13,600·10−6 cm3/mol | |
| Structure | |
| Rhombohedral, hP3, SpaceGroup = P-3m1, No. 164 | |
| octahedral | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
none |
| Related compounds | |
Other anions
|
Iron(II) fluoride Iron(II) chloride Iron(II) iodide |
Other cations
|
Manganese(II) bromide Cobalt(II) bromide |
Related compounds
|
Vanadium(II) bromide Iron(III) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

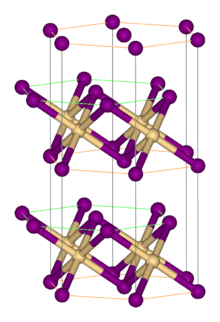
Structure
editLike most metal halides, FeBr2 adopts a polymeric structure consisting of isolated metal centers cross-linked with halides. It crystallizes with the CdI2 structure, featuring close-packed layers of bromide ions, between which are located Fe(II) ions in octahedral holes.[1] The packing of the halides is slightly different from that for FeCl2, which adopts the CdCl2 motif. The tetrahydrates FeX2(H2O)4 (X = Cl, Br) have similar structures, with octahedral metal centers and mutually trans halides.[2]
Synthesis and reactions
editFeBr2 is synthesized using a methanol solution of concentrated hydrobromic acid and iron powder. It adds the methanol solvate [Fe(MeOH)6]Br2 together with hydrogen gas. Heating the methanol complex in a vacuum gives pure FeBr2.[3]
FeBr2 reacts with two equivalents of tetraethylammonium bromide to give [(C2H5)4N]2FeBr4.[4] FeBr2 reacts with bromide and bromine to form the intensely colored, mixed-valence species [FeBr3Br9]−.[5]
Magnetism
editFeBr2 possesses a strong metamagnetism at 4.2 K and has long been studied as a prototypical metamagnetic compound.[6][7]
References
edit- ^ Haberecht, J.; Borrmann, Η.; Kniep, R. (2001). "Refinement of the crystal structure of iron dibromide, FeBr2". Zeitschrift für Kristallographie - New Crystal Structures. 216 (1–4). doi:10.1524/ncrs.2001.216.14.544.
- ^ Waizumi, Kenji; Masuda, Hideki; Ohtaki, Hitoshi (1992). "X-ray Structural Studies of FeBr2·4H2O, CoBr2·4H2O, NiCl2·4H2O and CuBr2·4H2O. Cis/Trans Selectivity in Transition Metal(II) Dihalide Tetrahydrate". Inorganica Chimica Acta. 192 (2): 173–181. doi:10.1016/S0020-1693(00)80756-2.
- ^ Winter, G. (1973). "Iron(II) Halides". Inorganic Syntheses. Vol. 14. pp. 99–104. doi:10.1002/9780470132456.ch20. ISBN 9780470132456.
- ^ N. S. Gill, F.. B. Taylor Inorganic Syntheses 1967, volume 9, page 136-142. doi:10.1002/9780470132401.ch37
- ^ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5
- ^ Wilkinson, M. K.; Cable, J. W.; Wollan, E. O.; Koehler, W. C. (15 January 1959). "Neutron Diffraction Investigations of the Magnetic Ordering in FeBr2, CoBr2, FeCl2, and CoCl2". Physical Review. 113 (2): 497–507. Bibcode:1959PhRv..113..497W. doi:10.1103/PhysRev.113.497.
- ^ Jacobs, I. S.; Lawrence, P. E. (10 December 1967). "Metamagnetic Phase Transitions and Hysteresis in FeCl2". Physical Review. 164 (2): 866–878. Bibcode:1967PhRv..164..866J. doi:10.1103/PhysRev.164.866.