Mercury(II) thiocyanate (Hg(SCN)2) is an inorganic chemical compound, the coordination complex of Hg2+ and the thiocyanate anion. It is a white powder. It will produce a large, winding "snake" when ignited, an effect known as the Pharaoh's serpent.[2]
| |

| |
| Names | |
|---|---|
| Other names
Mercuric thiocyanate
Mercuric sulfocyanate | |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.008.886 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Hg(SCN)2 | |
| Molar mass | 316.755 g/mol |
| Appearance | White monoclinic powder |
| Odor | odorless |
| Density | 3.71 g/cm3, solid |
| Melting point | 165 °C (329 °F; 438 K) (decomposes) |
| 0.069 g/100 mL | |
| Solubility | Soluble in dilute hydrochloric acid, KCN, ammonia slightly soluble in alcohol, ether |
| −96.5·10−6 cm3/mol | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
highly toxic |
| GHS labelling:[1] | |
  
| |
| Danger | |
| H300, H310, H330, H373, H410 | |
| P260, P262, P270, P271, P273, P280, P284, P301+P316, P302+P352, P304+P340, P316, P319, P320, P321, P330, P361, P364, P391, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
46 mg/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis and structure
editThe first synthesis of mercury thiocyanate was probably completed in 1821 by Jöns Jacob Berzelius:
- HgO + 2 HSCN → Hg(SCN)2 + H2O
Evidence for the first pure sample was presented in 1866 prepared by a chemist named Otto Hermes.[2] It is prepared by treating solutions containing mercury(II) and thiocyanate ions. The low solubility product of mercury thiocyanate causes it to precipitate from the solution.[3] Most syntheses are achieved by precipitation:
- Hg(NO3)2 + 2 KSCN → Hg(SCN)2 + 2 KNO3
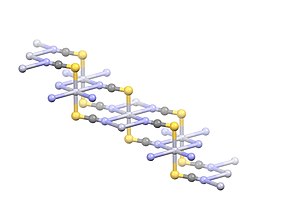
The compound adopts a polymeric structure with Hg2+ centres linearly coordinated to two S atoms with a distance of 2.381 Å. Four weak Hg2+--N interactions are indicated with distances of 2.81 Å.[4]
Reactions
editMercury thiocyanate has a few uses in chemical synthesis. It is the precursor to other thiocyanate complexes such as potassium tris(thiocyanato)mercurate(II) (K[Hg(SCN)3]) and caesium tris(thiocyanato)mercurate(II) (Cs[Hg(SCN)3]). The Hg(SCN)3− ion can also exist independently and is easily generated from the compounds above, amongst others.[5]
Its reactions with organic halides yield two products, one with the sulfur bound to the organic compound and one with the nitrogen bound to the organic compound.[6]
Use in chloride analysis
editMMercury thiocyanate improve detection limits in the determination of chloride ions in water by UV-visible spectroscopy. This technique was a standard method for the determination of chloride ions in laboratories worldwide. The method involves the addition of mercury thiocyanate to a solution with an unknown concentration of chloride ions and iron as a reagent. The chloride ions cause the mercury thiocyanate salt to dissociate and the thiocyanate ion to bind Fe(III), which absorbs intensely at 450 nm. This absorption allows for the measurement of the concentration of the iron complex. This value allows one to calculate the concentration of chloride.[7]
Pharaoh's serpent
editMercury thiocyanate was formerly used in pyrotechnics causing an effect known as the Pharaoh's serpent or Pharaoh's snake. The fireworks versions were a mixture with a small amount of potassium nitrate and gum arabic as a binder.[8] This use ceased in most countries in the early 20th century due to the toxicity of mercury, and the existence of a superior alternatives.
Chemistry of the reaction
editWhen heated, mercury(II) thiocyanate decomposes in an exothermic reaction that can produce a large mass of coiling, serpent-like solid. An inconspicuous flame, which is usually the blue color of burning carbon disulfide but which can be yellow from impurities or incidental combustion of flammable materials on the surface it is ignited on. The resulting solid can range from dark graphite gray to light tan in colour with the inside generally much darker than the outside. This was found to be due to decomposition of the produced β-HgS (black mercury sulfide) and vaporization of the resulting mercury from the othermost and hottest layers of the solid.[9]
The decomposition of Hg(SCN)2 is exothermic on its own, and the CS2 produced ignites easily and burns off. The C3N4 product is a simplification; the actual product contains 0.5% hydrogen and is likely to consist of sheets of triazine rings linked by −N= and −NH− groups similar to g−C3N4 and was found to contain nano-particles of β-HgS (black mercury sulfide).[9]
The number of resonance structures of heptazine and triazine, varying molecular weights of samples, and the fluorescense of the product made acquiring spectra difficult even by relatively exotic methods of NMR (with one spectrum acquisition being run for 12 days straight to get a mostly clean reading). Because of this, a heptazine-based structure similar to Liebig's melon, a compound initially prepared around the same time that the pharoah's snake reaction was discovered, was not ruled out by the authors as a partial component of the solid material.[9] The generalized reaction is as follows:
- 2 Hg(SCN)2 → 2 β−HgS + CS2 + C3N4
- β−HgS + O2 → Hg + SO2 (not all mercury sulfide decomposes)
C3N4 is not a product of this decomposition. Cyanogen is generally only produced when Hg(CN)2 or similar is heated to decomposition, and early attempts to form (SCN)2 via the same route starting at this compound failed and only generated SO2, CO2 and N2.[9]
References
edit- ^ "Mercuric thiocyanate (Compound)". pubchem.ncbi.nlm.nih.gov. Retrieved 31 May 2023.
- ^ a b Davis, T. L. (1940). "Pyrotechnic Snakes". Journal of Chemical Education. 17 (6): 268–270. doi:10.1021/ed017p268.
- ^ Sekine, T.; Ishii, T. (1970). "Studies of the Liquid-Liquid Partition systems. VIII. The Solvent Extraction of Mercury (II) Chloride, Bromide, Iodide and Thiocyanate with Some Organic Solvents". Bulletin of the Chemical Society of Japan. 43 (8): 2422–2429. doi:10.1246/bcsj.43.2422.
- ^ Beauchamp, A.L.; Goutier, D. "Structure cristalline et moleculaire du thiocyanate mercuric" Canadian Journal of Chemistry 1972, volume 50, p977-p981. doi:10.1139/v72-153
- ^ Bowmaker, G. A.; Churakov, A. V.; Harris, R. K.; Howard, J. A. K.; Apperley, D. C. (1998). "Solid-State 199Hg MAS NMR Studies of Mercury(II) Thiocyanate Complexes and Related Compounds. Crystal Structure of Hg(SeCN)2". Inorganic Chemistry. 37 (8): 1734–1743. doi:10.1021/ic9700112.
- ^ Kitamura, T.; Kobayashi, S.; Taniguchi, H. (1990). "Photolysis of Vinyl Halides. Reaction of Photogenerated Vinyl Cations with Cyanate and Thiocyanate Ions". Journal of Organic Chemistry. 55 (6): 1801–1805. doi:10.1021/jo00293a025.
- ^ Cirello-Egamino, J.; Brindle, I. D. (1995). "Determination of chloride ions by reaction with mercury thiocyanate in the absence of iron(III) using a UV-photometric, flow injection method". Analyst. 120 (1): 183–186. doi:10.1039/AN9952000183.
- ^ Weingart, George W. (1947). "Part III. Products of Manufacture and Formulas". Pyrotechnics (2d, rev. and enl ed.). Brooklyn: Chemical Pub. Co. pp. 182–183. ISBN 9780820601120.
- ^ a b c d Miller, Thomas S.; d'Aleo, Anita; Suter, Theo; Aliev, Abil E.; Sella, Andrea; McMillan, Paul F. (17 November 2017). "Pharaoh's Serpents: New Insights into a Classic Carbon Nitride Material". Zeitschrift für anorganische und allgemeine Chemie (Journal of Inorganic and General Chemistry). 643 (21): 1572–1580. doi:10.1002/zaac.201700268.
External links
edit- "Pharaoh's snake". YouTube. September 2, 2008.
