A motivation-enhancing drug,[2][3] also known as a pro-motivational drug,[1] is a drug which increases motivation.[4][1] Drugs enhancing motivation can be used in the treatment of motivational deficits, for instance in depression, schizophrenia, and attention deficit hyperactivity disorder (ADHD).[5][4] They can also be used in the treatment of disorders of diminished motivation (DDMs), including apathy, abulia, and akinetic mutism, disorders that can be caused by conditions like stroke, traumatic brain injury (TBI), and neurodegenerative diseases.[6][7] Motivation-enhancing drugs are used non-medically by healthy people to increase motivation and productivity as well, for instance in educational contexts.[8][1][9][10]
| Motivation-enhancing drug | |
|---|---|
| Drug class | |
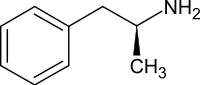 Dextroamphetamine, one of the most widely used motivation-enhancing drugs. | |
| Class identifiers | |
| Synonyms | Motivation-enhancing agent; Motivation-enhancing medication; Pro-motivational drug;[1] Pro-motivational agent; Pro-motivational medication |
| Use | To increase motivation and treat disorders of diminished motivation |
| Legal status | |
| In Wikidata | |
There are limited clinical data on medications in treating motivational deficits and disorders.[11][12] In any case, drugs used for pro-motivational purposes are generally dopaminergic agents, for instance dopamine reuptake inhibitors (DRIs) like methylphenidate and modafinil, dopamine releasing agents (DRAs) like amphetamine, and other dopaminergic medications.[4][1][13] Adenosine receptor antagonists, like caffeine and istradefylline, can also produce pro-motivational effects.[13][14][15][16] Acetylcholinesterase inhibitors, like donepezil, have been used as well.[17][18][6][11]
Some drugs do not appear to increase motivation and can actually have anti-motivational effects.[4][13][19] Examples of these drugs include selective serotonin reuptake inhibitors (SSRIs),[19][20][21] selective norepinephrine reuptake inhibitors (NRIs),[19] and antipsychotics (which are dopamine receptor antagonists or partial agonists).[22][23][24][25] Cannabinoids, for instance those found in cannabis, have also been associated with motivational deficits.[26][27][28][4][29]
Types of motivation-enhancing drugs
editDopaminergic agents
editDopaminergic agents that have been found to produce pro-motivational effects in animals and/or humans include the following:[4][13]
- Dopamine reuptake inhibitors (DRIs) like bupropion, CE-123, CE-158, CT-005404, JJC8-088, JJC8-089, methylphenidate, (S)-MK-26, modafinil, MRZ-9547 ((R)-phenylpiracetam), nomifensine, PRX-14040, pyrovalerone, RDS03-94, and vanoxerine (GBR-12909)[4][13][30]
- Dopamine releasing agents (DRAs) like amphetamine and lisdexamfetamine[4][31]
- Dopamine D1 receptor agonists like razpipadon[32][33][34]
- Dopamine precursors like levodopa (L-DOPA)[32][17][35]
- Catecholaminergic activity enhancers (CAEs) like selegiline, PPAP, and BPAP[36][37][38][39]
Other dopaminergic agents
editDopamine D2-like receptor agonists, including pramipexole, ropinirole, rotigotine, piribedil, bromocriptine, cabergoline, pergolide, and lisuride, have also been used to treat disorders of diminished motivation in humans.[18][6][7][12][40][41][42] The clinical data on these agents for this use is very limited, but therapeutic successes have been reported.[12][41] D2-like receptor agonists are known to have sedative-like and non-rewarding effects in humans.[43][44][45] In any case, dopamine D2-like receptor antagonists, like haloperidol and other antipsychotics, are known to produce anti-motivational effects in animals[4][13][12][1] and humans.[22][23][46][47][48][49] Bromocriptine has been reported to improve anergia and motivation in humans in very limited clinical reports.[40][50][51] On the other hand, pergolide failed to show pro-motivational effects in animals.[52]
Other dopaminergic drugs that have been used or suggested in the treatment of disorders of diminished motivation include rasagiline (a selective monoamine oxidase B (MAO-B) inhibitor; but see more below), tolcapone (a centrally-acting catechol-O-methyltransferase (COMT) inhibitor), and amantadine (an indirectly acting dopaminergic agent that acts via unknown mechanisms).[12][18][53][17][54] Tolcapone, the only marketed COMT inhibitor that is centrally acting (as opposed to peripherally selective), shows antidepressant- and anti-anhedonia-like effects, stimulates exploratory behavior, and enhances the locomotor hyperactivity induced by psychostimulants like amphetamine and nomifensine in animals.[55][56][57] Amantadine is widely used to treat multiple sclerosis-related fatigue, among other fatigue- and motivation-related disorders, and is recommended by the United Kingdom National Institute for Health and Care Excellence (NICE) guidelines for this use, although clinical data are limited.[54][58][59][60][61]
Mechanistic aspects of specific dopaminergic agents
editDopamine levels and signaling in the nucleus accumbens, part of the ventral striatum and the mesolimbic reward pathway, are thought to play a key role in mediating behavioral activation and motivation.[4][19][13][12] Dopamine releasing agents like dextroamphetamine are able to rapidly increase striatal dopamine levels by 700 to 1,500% of baseline in rodents.[62] These drugs show greater magnitudes of impact on dopamine levels than do dopamine reuptake inhibitors like methylphenidate.[62][63] In addition, whereas dopamine reuptake inhibitors show a clear dose–effect ceiling in their effects on dopamine levels, dopamine releasing agents do not and have been found to maximally increase dopamine levels by more than 5,000%.[62][64] Atypical dopamine reuptake inhibitors like modafinil can also increase dopamine levels in the striatum and nucleus accumbens in animals, but have further reduced impacts on dopamine levels compared to psychostimulants like amphetamine and methylphenidate.[65]
Limitations of specific dopaminergic agents
editA limitation of certain dopaminergic medications used to improve motivation, like psychostimulants, is development of tolerance to their effects.[66][67] Rapid acute tolerance to amphetamines is believed to be responsible for the dissociation between their relatively short durations of action (~4 hours for main desired effects) and their much longer elimination half-lives (~10 hours) and durations in the body (~2 days).[67][68][69][70][71][72][73] It appears that continually increasing or ascending concentration–time curves are beneficial for prolonging effects, which has resulted in administration multiple times per day and development of delayed- and extended-release formulations.[67][69][70] Drug holidays and breaks can be helpful in resetting tolerance.[66]
Another possible limitation of amphetamine specifically is dopaminergic neurotoxicity, which might occur even at therapeutic doses.[74][75][76][77][78][79]
A limitation of bupropion as a dopaminergic agent is that it achieves very limited clinical occupancy of the dopamine transporter (DAT).[80][81][82][83]
Adenosinergic agents
editAdenosine receptor antagonists, including caffeine, istradefylline (KW-6002), Lu AA47070, MSX-3, MSX-4, preladenant (SCH-420814), and theophylline, have shown pro-motivational effects in animals and humans.[13][14][15][84][16][85] Caffeine and theophylline act as non-selective antagonists of the adenosine receptors (including A1, A2A, A2B, and A3).[13][86][87][88] Conversely, agents like istradefylline and preladenant are selective adenosine A2A receptor antagonists.[13] Adenosine A2A receptor antagonists, including the non-selective antagonists like caffeine, show pro-motivational effects in animals, whereas selective adenosine A1 receptor antagonists, like DPCPX and CPX, do not.[13][89] Adenosine A2A receptor antagonists appear to exert their pro-motivational effects in the nucleus accumbens core and can reverse the anti-motivational effects of dopamine D2 receptor antagonists like haloperidol in animals.[13][14][15][90][91] Istradefylline is approved in the treatment of Parkinson's disease and has been found to improve symptoms of apathy, anhedonia, and depression in people with the condition.[16][85]
Cholinergic agents
editAcetylcholinesterase inhibitors, like donepezil, rivastigmine, and galantamine, have been used in the treatment of disorders of diminished motivation.[17][18][6][11] These drugs inhibit acetylcholinesterase, which metabolizes the neurotransmitter acetylcholine, thereby increasing acetylcholine levels in the brain and augmenting activation of the muscarinic acetylcholine and nicotinic acetylcholine receptors.[92] They are approved and used in the treatment of Alzheimer's disease and provide modest cognitive improvements in people with the disease.[92][93][94] Although acetylcholinesterase inhibitors have been used to treat disorders of diminished motivation, the muscarinic acetylcholine receptor agonist pilocarpine has actually shown anti-motivational effects in animals that can be reversed by the muscarinic acetylcholine receptor antagonist scopolamine.[90] In addition, xanomeline, a muscarinic acetylcholine M1 and M4 receptor agonist, shows indirect antidopaminergic effects in the mesolimbic pathway in animals and, in combination with trospium, is approved as an antipsychotic in the treatment of schizophrenia.[95][96][97] Furthermore, scopolamine has been found to reverse the anti-motivational effects of the dopamine D2 receptor antagonist haloperidol in animals.[90] In any case, in spite of the preceding findings, acetylcholinesterase inhibitors have been found to be clinically effective, albeit modestly, for apathy in dementia and Parkinson's disease.[98][99][100]
Other agents
editAgomelatine, a serotonin 5-HT2C receptor antagonist and melatonin MT1 and MT2 receptor agonist that has sometimes been described as a "norepinephrine–dopamine disinhibitor" ("NDDI") (in the prefrontal cortex),[101] has indirect dopaminergic actions and has been suggested as a possible treatment for disorders of diminished motivation like anhedonia and abulia.[102] It has been found to be effective in the treatment of apathy in people with dementia.[103][98][104][105] The drug was also reported to reverse escitalopram-associated apathy in a case report.[102][106]
The GPR139 agonist zelatriazin (TAK-041; NBI-1065846) has shown pro-motivational effects in animals.[107][108] On the basis of these findings, it has been speculated that the drug might be useful in the treatment of apathy in humans.[107][108] Zelatriazin was under development for the treatment of anhedonia in major depressive disorder and the negative symptoms of schizophrenia and reached phase 3 clinical trials.[109][110][111] However, its development was discontinued due to lack of clinical effectiveness.[109][112]
The tumor necrosis factor α (TNF-α) monoclonal antibody infliximab has been found to increase motivation in people with depression with high inflammation (as measured by high C-reactive protein levels).[113][114] The drug has also been found to reduce symptoms of depression and anhedonia, for instance in people with high inflammation.[115][116][113]
Ineffective agents
editSerotonergic and noradrenergic agents
editSelective serotonin reuptake inhibitors (SSRIs) like escitalopram and norepinephrine reuptake inhibitors (NRIs) like atomoxetine have been used and recommended in the treatment of disorders of diminished motivation.[7][17][117] However, SSRIs like fluoxetine and citalopram, NRIs like desipramine and atomoxetine, and MAO-A-inhibiting monoamine oxidase inhibitors (MAOIs) like moclobemide and pargyline, have all not shown pro-motivational effects in animals.[4][13][30][118][39] In fact, these drugs can produce further motivational deficits in animals.[19][118][119][39] Serotonergic antidepressants like SSRIs and serotonin–norepinephrine reuptake inhibitors (SNRIs) have also been implicated in inducing apathy and emotional blunting in humans.[20][21][120]
Selective MAO-B inhibitors
editIn contrast to selegiline, selective MAO-B inhibitors without concomitant catecholaminergic activity enhancer (CAE) actions, like rasagiline, SU-11739, and lazabemide, are poorly effective in reversing behavioral deficits induced by the dopamine depleting agent tetrabenazine in animals.[121][122]
Dopamine receptor antagonists and partial agonists
editAntipsychotics, which classically act as dopamine receptor antagonists (mostly of the D2-like receptors), are well-known as having robust and dose-dependent anti-motivational effects.[4][13][22][23][46][47][49] In fact, these effects may play a key role in their effectiveness against the positive and psychotic symptoms of schizophrenia by blunting the emotions underlying delusions.[22][23][46][47][49]
A novel class of antipsychotics, sometimes referred to as third-generation antipsychotics, act as dopamine D2-like receptor partial agonists instead of as pure antagonists, and hence have mixed agonistic and antagonistic effects.[123][124] These drugs include aripiprazole, brexpiprazole, and cariprazine.[124] Aripiprazole has been suggested, at low doses, as a possible treatment for disorders of diminished motivation.[53] However, aripiprazole and cariprazine showed anti-motivational effects in animals and failed to reverse the motivational deficits induced by the dopamine depleting agent tetrabenazine.[25][24] Accordingly, aripiprazole reduced activation of the mesolimbic motivational pathway in humans similarly to but less robustly than haloperidol.[125][126] On the other hand, another study found that aripiprazole reversed stress-induced motivational anhedonia in animals, an antidepressant-like effect.[127][128] Different dopamine receptor partial agonists that are used in the treatment of schizophrenia are known to vary in their intrinsic activities at the dopamine receptors, so each drug may be expected to have a different profile of effects.[129]
Certain atypical dopamine reuptake inhibitors
editSome atypical DRIs, like JJC8-091, in contrast to other DRIs, are not effective in producing pro-motivational effects in animals.[130] This has been attributed to binding to an occluded conformation of the dopamine transporter (DAT) that results in a diminished increase in dopamine levels.[130]
See also
editReferences
edit- ^ a b c d e f Hailwood JM (27 September 2018). Novel Approaches Towards Pharmacological Enhancement of Motivation (Thesis). University of Cambridge. doi:10.17863/CAM.40216.
The ethical considerations of pharmacological enhancement of cognition in the healthy population have been debated elsewhere (Farah et al. 2004; Porsdam Mann & Sahakian 2015). It is likely that putative pro-motivational drugs deserve a similar level of scrutiny.
- ^ Zohny, Hazem (7 July 2015). "The Problem with Artificial Willpower". Scientific American. Retrieved 16 October 2024.
The ethical threat posed by Adderall and other drugs that improve motivation [...] If it isn't justified – that is, if her options are limited purely due to unjust socio-political forces – then motivation enhancing drugs start to look more like political complacence pills. [...] It's the sort of spectre that permeates dystopian visions of the future, and it's one that is very much raised by the prospect of motivation enhancing drugs.
- ^ Ray, Keisha Shantel (2 January 2015). "Motivation's Pick-Me-Upper: Enhancing Performance Through Motivation-Enhancing Drugs". AJOB Neuroscience. 6 (1). Informa UK Limited: 50–51. doi:10.1080/21507740.2014.999888. ISSN 2150-7740.
- ^ a b c d e f g h i j k l Salamone JD, Correa M (January 2024). "The Neurobiology of Activational Aspects of Motivation: Exertion of Effort, Effort-Based Decision Making, and the Role of Dopamine". Annu Rev Psychol. 75: 1–32. doi:10.1146/annurev-psych-020223-012208. hdl:10234/207207. PMID 37788571.
- ^ Salamone JD, Yohn SE, López-Cruz L, San Miguel N, Correa M (May 2016). "Activational and effort-related aspects of motivation: neural mechanisms and implications for psychopathology". Brain. 139 (Pt 5): 1325–1347. doi:10.1093/brain/aww050. PMC 5839596. PMID 27189581.
- ^ a b c d Spiegel DR, Warren A, Takakura W, Servidio L, Leu N (January 2018). "Disorders of diminished motivation: What they are, and how to treat them" (PDF). Current Psychiatry. 17 (1): 10–18, 20.
- ^ a b c Arnts H, van Erp WS, Lavrijsen JC, van Gaal S, Groenewegen HJ, van den Munckhof P (May 2020). "On the pathophysiology and treatment of akinetic mutism". Neuroscience and Biobehavioral Reviews. 112: 270–278. doi:10.1016/j.neubiorev.2020.02.006. hdl:2066/225901. PMID 32044373.
- ^ Kjærsgaard T (2 January 2015). "Enhancing Motivation by Use of Prescription Stimulants: The Ethics of Motivation Enhancement". AJOB Neuroscience. 6 (1): 4–10. doi:10.1080/21507740.2014.990543. ISSN 2150-7740.
- ^ Sharif S, Guirguis A, Fergus S, Schifano F (March 2021). "The Use and Impact of Cognitive Enhancers among University Students: A Systematic Review". Brain Sci. 11 (3): 355. doi:10.3390/brainsci11030355. PMC 8000838. PMID 33802176.
- ^ Brühl AB, d'Angelo C, Sahakian BJ (2019). "Neuroethical issues in cognitive enhancement: Modafinil as the example of a workplace drug?". Brain Neurosci Adv. 3: 2398212818816018. doi:10.1177/2398212818816018. PMC 7058249. PMID 32166175.
- ^ a b c Starkstein SE, Pahissa J (2018). "Disorders of Diminished Motivation". In Silver JM, McAllister TW, Arciniegas DB (eds.). Textbook of Traumatic Brain Injury (3 ed.). American Psychiatric Association Publishing. pp. 381–393. ISBN 978-1-61537-112-9. Retrieved 17 September 2024.
- ^ a b c d e f Chong TT, Husain M (2016). "The role of dopamine in the pathophysiology and treatment of apathy". Motivation: Theory, Neurobiology and Applications. Progress in Brain Research. Vol. 229. pp. 389–426. doi:10.1016/bs.pbr.2016.05.007. ISBN 978-0-444-63701-7. PMID 27926449.
- ^ a b c d e f g h i j k l m n Salamone JD, Correa M, Ferrigno S, Yang JH, Rotolo RA, Presby RE (October 2018). "The Psychopharmacology of Effort-Related Decision Making: Dopamine, Adenosine, and Insights into the Neurochemistry of Motivation". Pharmacol Rev. 70 (4): 747–762. doi:10.1124/pr.117.015107. PMC 6169368. PMID 30209181.
- ^ a b c Treadway MT, Salamone JD (2022). "Vigor, Effort-Related Aspects of Motivation and Anhedonia". Curr Top Behav Neurosci. Current Topics in Behavioral Neurosciences. 58: 325–353. doi:10.1007/7854_2022_355. ISBN 978-3-031-09682-2. PMID 35505057.
Adenosine A2A receptor antagonists have been studied for their potential antiparkinsonian effects (Ferré 1997; Morelli and Pinna 2002; Correa et al. 2004), and istradefylline (Nourianz) has been approved for use in several countries. Particularly relevant for the present review, drugs that act on adenosine A2A receptors induce substantial effects on instrumental behavior and effort-related choice. [...] Caffeine, theophylline, and several adenosine A2A receptor antagonists (MSX-3, MSX-4, Lu AA47070, istradefylline) can reverse the low-effort bias induced by systemically administered DA D2 antagonists (Farrar et al. 2007; Worden et al. 2009; Mott et al. 2009; Collins et al. 2012; Nunes et al. 2010; Santerre et al. 2012; Randall et al. 2012; Pardo et al. 2020), and MSX-3 and preladenant reverse the effects of TBZ (Nunes et al. 2013; Randall et al. 2014; Yohn et al. 2015a; Salamone et al. 2018). [...] Furthermore, A2A receptor knockout mice are resistant to the effort-related effects of haloperidol (Pardo et al. 2012). [...] Along with adenosine A2A antagonists such as istradefylline and preladenant (Nunes et al. 2013; Randall et al. 2014; Yohn et al. 2015a; Salamone et al. 2018), and D1 agonists (Yohn et al. 2015b), atypical DAT inhibitors offer promise as potential treatments for effort-related motivational symptoms.
- ^ a b c Ferré S, Díaz-Ríos M, Salamone JD, Prediger RD (December 2018). "New Developments on the Adenosine Mechanisms of the Central Effects of Caffeine and Their Implications for Neuropsychiatric Disorders". J Caffeine Adenosine Res. 8 (4): 121–131. doi:10.1089/caff.2018.0017. PMC 6306650. PMID 30596206.
- ^ a b c López-Cruz L, Salamone JD, Correa M (2018). "Caffeine and Selective Adenosine Receptor Antagonists as New Therapeutic Tools for the Motivational Symptoms of Depression". Front Pharmacol. 9: 526. doi:10.3389/fphar.2018.00526. PMC 5992708. PMID 29910727.
- ^ a b c d e Krishnamoorthy A, Craufurd D (October 2011). "Treatment of Apathy in Huntington's Disease and Other Movement Disorders". Curr Treat Options Neurol. 13 (5): 508–519. doi:10.1007/s11940-011-0140-y. PMID 21800056.
- ^ a b c d Marin RS, Wilkosz PA (2005). "Disorders of diminished motivation". The Journal of Head Trauma Rehabilitation. 20 (4): 377–388. doi:10.1097/00001199-200507000-00009. PMID 16030444.
- ^ a b c d e Salamone JD, Pardo M, Yohn SE, López-Cruz L, SanMiguel N, Correa M (2016). "Mesolimbic Dopamine and the Regulation of Motivated Behavior". Curr Top Behav Neurosci. Current Topics in Behavioral Neurosciences. 27: 231–257. doi:10.1007/7854_2015_383. ISBN 978-3-319-26933-7. PMID 26323245.
- ^ a b Jawad MY, Fatima M, Hassan U, Zaheer Z, Ayyan M, Ehsan M, Khan MH, Qadeer A, Gull AR, Asif MT, Shad MU (July 2023). "Can antidepressant use be associated with emotional blunting in a subset of patients with depression? A scoping review of available literature". Human Psychopharmacology. 38 (4): e2871. doi:10.1002/hup.2871. PMID 37184083.
- ^ a b Masdrakis VG, Markianos M, Baldwin DS (August 2023). "Apathy associated with antidepressant drugs: a systematic review". Acta Neuropsychiatrica. 35 (4): 189–204. doi:10.1017/neu.2023.6. PMID 36644883.
- ^ a b c d Thompson J, Stansfeld JL, Cooper RE, Morant N, Crellin NE, Moncrieff J (February 2020). "Experiences of taking neuroleptic medication and impacts on symptoms, sense of self and agency: a systematic review and thematic synthesis of qualitative data". Soc Psychiatry Psychiatr Epidemiol. 55 (2): 151–164. doi:10.1007/s00127-019-01819-2. PMID 31875238.
- ^ a b c d Belmaker, Robert Haim; Lichtenberg, Pesach (2023). "Antipsychotic Drugs: Do They Define Schizophrenia or Do They Blunt All Emotions?". Psychopharmacology Reconsidered: A Concise Guide Exploring the Limits of Diagnosis and Treatment. Cham: Springer International Publishing. pp. 63–84. doi:10.1007/978-3-031-40371-2_6. ISBN 978-3-031-40370-5.
- ^ a b Ecevitoglu A, Edelstein GA, Presby RE, Rotolo RA, Yang JH, Quiles T, Okifo K, Conrad RT, Kovach A, Correa M, Salamone JD (August 2023). "Effects of the atypical antipsychotic and D3/D2 dopamine partial agonist cariprazine on effort-based choice behavior: implications for modeling avolition". Psychopharmacology (Berl). 240 (8): 1747–1757. doi:10.1007/s00213-023-06405-8. PMID 37358806.
- ^ a b Mitola, Matthew (2023). "Assessing the Impact of Aripiprazole on Effort-Based Decision Making in Mice Using Touchscreen Procedures". CT Digital Archive. Retrieved 26 September 2024.
- ^ Skumlien M, Langley C, Lawn W, Voon V, Curran HV, Roiser JP, Sahakian BJ (November 2021). "The acute and non-acute effects of cannabis on reward processing: A systematic review". Neuroscience and Biobehavioral Reviews. 130: 512–528. doi:10.1016/j.neubiorev.2021.09.008. PMID 34509513.
- ^ Pacheco-Colón I, Limia JM, Gonzalez R (August 2018). "Nonacute effects of cannabis use on motivation and reward sensitivity in humans: A systematic review". Psychology of Addictive Behaviors. 32 (5): 497–507. doi:10.1037/adb0000380. PMC 6062456. PMID 29963875.
- ^ Skumlien M, Langley C, Sahakian BJ (19 December 2023). "Is Cannabis Use Associated with Motivation? A Review of Recent Acute and Non-Acute Studies". Current Behavioral Neuroscience Reports. 11: 33–43. doi:10.1007/s40473-023-00268-1. ISSN 2196-2979.
- ^ Silveira MM, Adams WK, Morena M, Hill MN, Winstanley CA (March 2017). "Δ9-Tetrahydrocannabinol decreases willingness to exert cognitive effort in male rats". J Psychiatry Neurosci. 42 (2): 131–138. doi:10.1503/jpn.150363. PMC 5373702. PMID 28245177.
- ^ a b Goldhamer A (2023). "The Role of Dopamine in Effort- Based Decisions: Insights from Bupropion, Nomifensine, and Atomoxetine". CT Digital Archive. Retrieved 16 September 2024.
- ^ Yohn SE, Lopez-Cruz L, Hutson PH, Correa M, Salamone JD (March 2016). "Effects of lisdexamfetamine and s-citalopram, alone and in combination, on effort-related choice behavior in the rat". Psychopharmacology (Berl). 233 (6): 949–960. doi:10.1007/s00213-015-4176-7. PMID 26694811.
- ^ a b Webber HE, Lopez-Gamundi P, Stamatovich SN, de Wit H, Wardle MC (January 2021). "Using pharmacological manipulations to study the role of dopamine in human reward functioning: A review of studies in healthy adults". Neurosci Biobehav Rev. 120: 123–158. doi:10.1016/j.neubiorev.2020.11.004. PMC 7855845. PMID 33202256.
Similarly, augmenting DA using a D1 agonist (PF-06412562; 6mg, 15mg, 30mg) increased willingness to exert physical effort for reward (Soutschek et al., 2020).
- ^ Soutschek A, Gvozdanovic G, Kozak R, Duvvuri S, de Martinis N, Harel B, Gray DL, Fehr E, Jetter A, Tobler PN (April 2020). "Dopaminergic D1 Receptor Stimulation Affects Effort and Risk Preferences". Biol Psychiatry. 87 (7): 678–685. doi:10.1016/j.biopsych.2019.09.002. PMID 31668477.
- ^ Yohn SE, Santerre JL, Nunes EJ, Kozak R, Podurgiel SJ, Correa M, Salamone JD (August 2015). "The role of dopamine D1 receptor transmission in effort-related choice behavior: Effects of D1 agonists". Pharmacol Biochem Behav. 135: 217–226. doi:10.1016/j.pbb.2015.05.003. PMID 26022661.
- ^ Zénon A, Devesse S, Olivier E (September 2016). "Dopamine Manipulation Affects Response Vigor Independently of Opportunity Cost". J Neurosci. 36 (37): 9516–9525. doi:10.1523/JNEUROSCI.4467-15.2016. PMC 6601940. PMID 27629704.
- ^ Knoll J (2001). "Antiaging compounds: (-)deprenyl (selegeline) and (-)1-(benzofuran-2-yl)-2-propylaminopentane, [(-)BPAP], a selective highly potent enhancer of the impulse propagation mediated release of catecholamine and serotonin in the brain". CNS Drug Reviews. 7 (3): 317–345. doi:10.1111/j.1527-3458.2001.tb00202.x. PMC 6494119. PMID 11607046.
- ^ Knoll J (August 2003). "Enhancer regulation/endogenous and synthetic enhancer compounds: a neurochemical concept of the innate and acquired drives". Neurochemical Research. 28 (8): 1275–1297. doi:10.1023/a:1024224311289. PMID 12834268.
- ^ Randall PA, Lee CA, Nunes EJ, Yohn SE, Nowak V, Khan B, Shah P, Pandit S, Vemuri VK, Makriyannis A, Baqi Y, Müller CE, Correa M, Salamone JD (2014). "The VMAT-2 inhibitor tetrabenazine affects effort-related decision making in a progressive ratio/chow feeding choice task: reversal with antidepressant drugs". PLOS ONE. 9 (6): e99320. Bibcode:2014PLoSO...999320R. doi:10.1371/journal.pone.0099320. PMC 4061002. PMID 24937131.
- ^ a b c Contreras-Mora H, Rowland MA, Yohn SE, Correa M, Salamone JD (March 2018). "Partial reversal of the effort-related motivational effects of tetrabenazine with the MAO-B inhibitor deprenyl (selegiline): Implications for treating motivational dysfunctions". Pharmacol Biochem Behav. 166: 13–20. doi:10.1016/j.pbb.2018.01.001. PMID 29309800.
- ^ a b Salamone JD, Koychev I, Correa M, McGuire P (August 2015). "Neurobiological basis of motivational deficits in psychopathology". Eur Neuropsychopharmacol. 25 (8): 1225–1238. doi:10.1016/j.euroneuro.2014.08.014. PMID 25435083.
- ^ a b Sami MB, Faruqui R (December 2015). "The effectiveness of dopamine agonists for treatment of neuropsychiatric symptoms post brain injury and stroke". Acta Neuropsychiatr. 27 (6): 317–326. doi:10.1017/neu.2015.17. PMID 25850757.
Dopamine agonists have been reported to have positive effects in treating the neuropsychiatric sequalae of brain injury. One case series reported 19 out of 30 patients with severe head injury and aggression to respond to amantadine over the course of a year (15). Other case series have also shown positive response of cognitive function, attention and motivation in persons with head injury in the rehabilitative setting (16–18). Open label trials have shown improved neuropsychiatric outcomes in braininjured patients with bromocriptine and amantadine (19,20). Case studies have also reported improvement with the use of dopaminergic therapy in patients with neuropsychiatric sequalae of stroke. A combination of carbidopa/levodopa and pergolide has been reported to substantially improve the outcome of post-infarct akinetic mutism (21). Ropinirole has been reported to have had a dramatic affect on post-stroke apathy (22). However most of the reported associations to date have been limited by considerable methodological shortcomings. Case studies are anecdotal evidence, whereas larger case series may report improvement but are uncontrolled. This is critical in studies of neurological injury, where a degree of improvement may be expected by neuronal recovery over time. Similarly trials to date which have reported positive results have been open-label and consequently susceptible to placebo effect. Thus a systematic review of rigorous double-blind randomised controlled trials (RCTs) is needed.
- ^ Barrett K (August 1991). "Treating organic abulia with bromocriptine and lisuride: four case studies". J Neurol Neurosurg Psychiatry. 54 (8): 718–721. doi:10.1136/jnnp.54.8.718. PMC 1014478. PMID 1940945.
- ^ Rothman RB (1994). "A Review of the Effects of Dopaminergic Agents in Humans: Implications for Medication Development". In Erinoff L, Brown RM (eds.). Neurobiological Models for Evaluating Mechanisms Underlying Cocaine Addiction (NIDA Research Monograph 145) (PDF). U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute on Drug Abuse. pp. 67–87. Retrieved 4 August 2024.
- ^ Rothman RB, Glowa JR (1995). "A review of the effects of dopaminergic agents on humans, animals, and drug-seeking behavior, and its implications for medication development. Focus on GBR 12909". Mol Neurobiol. 11 (1–3): 1–19. doi:10.1007/BF02740680. PMID 8561954.
- ^ Singer C (January 2002). "Adverse effects in the treatment of Parkinson's disease". Expert Rev Neurother. 2 (1): 105–118. doi:10.1586/14737175.2.1.105. PMID 19811020.
- ^ a b c Moncrieff, Joanna (2007). "What Do Neuroleptics Really Do? A Drug-Centred Account". The Myth of the Chemical Cure: A Critique of Psychiatric Drug Treatment. Palgrave Macmillan London. pp. 100–117. doi:10.1007/978-0-230-58944-5_7 (inactive 1 November 2024). ISBN 978-0-230-57431-1.
{{cite book}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ a b c Moncrieff, Joanna (2013). "The Patient's Dilemma: Other Evidence on the Effects of Antipsychotics". The Bitterest Pills. London: Palgrave Macmillan UK. pp. 113–131. doi:10.1057/9781137277442_7. ISBN 978-1-137-27743-5.
- ^ Moncrieff J, Cohen D, Mason JP (August 2009). "The subjective experience of taking antipsychotic medication: a content analysis of Internet data". Acta Psychiatr Scand. 120 (2): 102–111. doi:10.1111/j.1600-0447.2009.01356.x. PMID 19222405.
- ^ a b c Healy D (October 1989). "Neuroleptics and psychic indifference: a review". J R Soc Med. 82 (10): 615–619. doi:10.1177/014107688908201018. PMC 1292340. PMID 2572700.
- ^ Brown AS, Gershon S (1993). "Dopamine and depression". J Neural Transm Gen Sect. 91 (2–3): 75–109. doi:10.1007/BF01245227. PMID 8099801.
- ^ Powell JH, al-Adawi S, Morgan J, Greenwood RJ (April 1996). "Motivational deficits after brain injury: effects of bromocriptine in 11 patients". J Neurol Neurosurg Psychiatry. 60 (4): 416–421. doi:10.1136/jnnp.60.4.416. PMC 1073895. PMID 8774407.
- ^ Marciano, Theresa (24 July 2023). "Assessing the effects of Pergolide on the motivational aspects of depression in rats using operant conditioning". Honors Scholar Theses. Retrieved 26 September 2024.
- ^ a b Costello H, Husain M, Roiser JP (January 2024). "Apathy and Motivation: Biological Basis and Drug Treatment". Annu Rev Pharmacol Toxicol. 64: 313–338. doi:10.1146/annurev-pharmtox-022423-014645. PMID 37585659.
- ^ a b Danysz W, Dekundy A, Scheschonka A, Riederer P (February 2021). "Amantadine: reappraisal of the timeless diamond-target updates and novel therapeutic potentials". J Neural Transm (Vienna). 128 (2): 127–169. doi:10.1007/s00702-021-02306-2. PMC 7901515. PMID 33624170.
- ^ Guay DR (January 1999). "Tolcapone, a selective catechol-O-methyltransferase inhibitor for treatment of Parkinson's disease". Pharmacotherapy. 19 (1): 6–20. doi:10.1592/phco.19.1.6.30516. PMID 9917075.
It also enhances locomotor hyperactivity induced by amphetamine and nomifensine and stereotypy induced by amphetamine, and stimulates exploratory activity in the open field test in rats and mice.14 Tolcapone potentiates levodopa antagonism of haloperidol-induced catalepsy in MPP+-lesioned mice (murine model of Parkinson's disease) and potentiates and prolongs levodopa-induced circling behavior in rats with 6-hydroxydopamine-induced nigrostriatal pathway lesions (another animal model of Parkinson's disease).23, 24 [...] The effect of tolcapone on animal models of depression was evaluated in two studies. In rats with chronic mild stress-induced anhedonia, tolcapone 10 or 30 mg/kg twice/day by intraperitoneal injection prevented the stress-induced anhedonic state compared with vehicle-treated controls.28 Another rat study using the forced swimming test and learned helplessness paradigm, found no significant antidepressant activity of the agent.29 The relevance of these findings to the management of depression in humans with both parkinsonian and nonparkinsonian disease is unknown.
- ^ Maj J, Rogóz Z, Skuza G, Sowińska H, Superata J (1990). "Behavioural and neurochemical effects of Ro 40-7592, a new COMT inhibitor with a potential therapeutic activity in Parkinson's disease". J Neural Transm Park Dis Dement Sect. 2 (2): 101–112. doi:10.1007/BF02260898. PMID 1977408.
- ^ Parada A, Soares-da-Silva P (October 2000). "POSTER COMMUNICATIONS: 49P. BIA 3-202 does not potentiate locomotor hyperactivity during increased dopaminergic stimulation". British Journal of Pharmacology. 131 (Suppl). Wiley: 38P–129P. PMC 1910551.
Tolcapone administered 6 h before amphetamine challenge was found to significantly increase locomotor activity in rats treated with 0.5 and 2.0 mg kg-1 amphetamine. In rats given 4.0 mg kg-1 amphetamine, tolcapone produced a marked decrease in locomotor activity and increased two-fold the duration of the stereotyped behaviour.
- ^ "Recommendations | Multiple sclerosis in adults: management | Guidance | NICE". www.nice.org.uk. June 22, 2022. Archived from the original on January 7, 2023. Retrieved January 7, 2023.
- ^ Zimek D, Miklusova M, Mares J (2023). "Overview of the Current Pathophysiology of Fatigue in Multiple Sclerosis, Its Diagnosis and Treatment Options - Review Article". Neuropsychiatr Dis Treat. 19: 2485–2497. doi:10.2147/NDT.S429862. PMC 10674653. PMID 38029042.
Currently, different pharmacological agents are used for the treatment for fatigue in patients with MS, including amantadine, modafinil and pemoline.99,100 Of these, the most commonly used is amantadine. Its main mechanism of action is not yet fully understood, although its effects on fatigue seem to be related to its dopaminergic effects, supporting the dopamine imbalance theory for MS-related fatigue.101 In general, all trials that compared amantadine with placebo showed a significant effect of amantadine on fatigue. However, the results of these trials need to be interpreted with caution because of the low number of participants included in the trials and the short duration of the interventions.8 The daily dose of amantadine used in all published studies was 200 mg, which is the standard amount administered today. Amantadine is the only oral treatment that is currently recommended by the National Institute for Health and Care Excellence (NICE) for the treatment of MS-related fatigue.102
- ^ Yang TT, Wang L, Deng XY, Yu G (September 2017). "Pharmacological treatments for fatigue in patients with multiple sclerosis: A systematic review and meta-analysis". J Neurol Sci. 380: 256–261. doi:10.1016/j.jns.2017.07.042. PMID 28870581.
- ^ Dobryakova E, Genova HM, DeLuca J, Wylie GR (2015). "The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders". Front Neurol. 6: 52. doi:10.3389/fneur.2015.00052. PMC 4357260. PMID 25814977.
- ^ a b c Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present--a pharmacological and clinical perspective". J Psychopharmacol. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- ^ Hodgkins P, Shaw M, Coghill D, Hechtman L (September 2012). "Amfetamine and methylphenidate medications for attention-deficit/hyperactivity disorder: complementary treatment options". Eur Child Adolesc Psychiatry. 21 (9): 477–492. doi:10.1007/s00787-012-0286-5. PMC 3432777. PMID 22763750.
Intraperitoneal administration of dl-threo-MPH 10 mg/kg to spontaneously hypertensive rats elicits a rapid 3–4-fold increase in extracellular concentrations of noradrenaline in the prefrontal cortex and dopamine in the striatum, peaking within 45 min of dosing, and remaining above control levels for at least 3 h [48]. [...] Intraperitoneal administration of d-AMF 1 mg/kg to spontaneously hypertensive rats elicits a 15-fold increase in striatal dopamine concentrations 30 min post-dose that return to control levels within 90 min, and a fourfold increase in noradrenaline concentrations in the prefrontal cortex within 45 min of dosing that remain above control levels for at least 3 h.
- ^ Cheetham SC, Kulkarni RS, Rowley HL, Heal DJ (2007). The SH rat model of ADHD has profoundly different catecholaminergic responses to amphetamine's enantiomers compared with Sprague-Dawleys. Neuroscience 2007, San Diego, CA, Nov 3-7, 2007. Society for Neuroscience. Archived from the original on 27 July 2024.
Both d- and l-[amphetamine (AMP)] evoked rapid increases in extraneuronal concentrations of [noradrenaline (NA)] and [dopamine (DA)] that reached a maximum 30 or 60 min after administration. However, the [spontaneously hypertensive rats (SHRs)] were much more responsive to AMP's enantiomers than the [Sprague-Dawleys (SDs)]. Thus, 3 mg/kg d-AMP produced a peak increase in [prefrontal cortex (PFC)] NA of 649 ± 87% (p<0.001) in SHRs compared with 198 ± 39% (p<0.05) in SDs; the corresponding figures for [striatal (STR)] DA were 4898 ± 1912% (p<0.001) versus 1606 ± 391% (p<0.001). At 9 mg/kg, l-AMP maximally increased NA efflux by 1069 ± 105% (p<0.001) in SHRs compared with 157 ± 24% (p<0.01) in SDs; the DA figures were 3294 ± 691% (p<0.001) versus 459 ± 107% (p<0.001).
- ^ Hersey M, Bacon AK, Bailey LG, Coggiano MA, Newman AH, Leggio L, Tanda G (2021). "Psychostimulant Use Disorder, an Unmet Therapeutic Goal: Can Modafinil Narrow the Gap?". Front Neurosci. 15: 656475. doi:10.3389/fnins.2021.656475. PMC 8187604. PMID 34121988.
MOD binding to DAT differs from that of other typical, cocaine-like, DAT blockers (Schmitt and Reith, 2011). In contrast to cocaine, MOD prefers to bind to, or stabilize the DAT protein in a more inward-facing occluded conformation (Schmitt and Reith, 2011; Loland et al., 2012) that still inhibits uptake and results in increases in extracellular DA in the NAcc (Ferraro et al., 1996c; Zolkowska et al., 2009), the NAcc shell (NAS) (Loland et al., 2012; Mereu et al., 2020), and the striatum (Rowley et al., 2014). MOD also increases electrically evoked DA in the DS and VS (Bobak et al., 2016) (summarized in Table 2) like abused psychostimulants (Nisell et al., 1994; Pontieri et al., 1996; Munzar et al., 2004; Kohut et al., 2014). However, while acute administration of MOD (Mereu et al., 2017, 2020) or its enantiomers (Loland et al., 2012; Keighron et al., 2019a, b) increases extracellular NAcc DA levels in rodents, these effects, even at very high doses, elicited a limited stimulation of DA in striatal areas compared to the stimulation elicited by abused psychostimulants (Loland et al., 2012; Mereu et al., 2017, 2020). This limited efficacy of MOD to increase DA levels, as compared to abused psychostimulants, also predicts a limited potential for abuse.
- ^ a b Handelman K, Sumiya F (July 2022). "Tolerance to Stimulant Medication for Attention Deficit Hyperactivity Disorder: Literature Review and Case Report". Brain Sciences. 12 (8): 959. doi:10.3390/brainsci12080959. PMC 9332474. PMID 35892400.
- ^ a b c Ermer JC, Pennick M, Frick G (May 2016). "Lisdexamfetamine Dimesylate: Prodrug Delivery, Amphetamine Exposure and Duration of Efficacy". Clinical Drug Investigation. 36 (5): 341–356. doi:10.1007/s40261-015-0354-y. PMC 4823324. PMID 27021968.
- ^ Cruickshank CC, Dyer KR (July 2009). "A review of the clinical pharmacology of methamphetamine". Addiction. 104 (7): 1085–1099. doi:10.1111/j.1360-0443.2009.02564.x. PMID 19426289.
Metabolism does not appear to be altered by chronic exposure, thus dose escalation appears to arise from pharmacodynamic rather than pharmacokinetic tolerance [24]. [...] The terminal plasma half-life of methamphetamine of approximately 10 hours is similar across administration routes, but with substantial inter-individual variability. Acute effects persist for up to 8 hours following a single moderate dose of 30 mg [30]. [...] peak plasma methamphetamine concentration occurs after 4 hours [35]. Nevertheless, peak cardiovascular and subjective effects occur rapidly (within 5–15 minutes). The dissociation between peak plasma concentration and clinical effects indicates acute tolerance, which may reflect rapid molecular processes such as redistribution of vesicular monoamines and internalization of monoamine receptors and transporters [6,36]. Acute subjective effects diminish over 4 hours, while cardiovascular effects tend to remain elevated. This is important, as the marked acute tachyphylaxis to subjective effects may drive repeated use within intervals of 4 hours, while cardiovascular risks may increase [11,35].
- ^ a b Abbas K, Barnhardt EW, Nash PL, Streng M, Coury DL (April 2024). "A review of amphetamine extended release once-daily options for the management of attention-deficit hyperactivity disorder". Expert Review of Neurotherapeutics. 24 (4): 421–432. doi:10.1080/14737175.2024.2321921. PMID 38391788.
For several decades, clinical benefits of amphetamines have been limited by the pharmacologic half-life of around 4 hours. Although higher doses can produce higher maximum concentrations, they do not affect the half-life of the dose. Therefore, to achieve longer durations of effect, stimulants had to be dosed at least twice daily. Further, these immediate-release doses were found to have their greatest effect shortly after administration, with a rapid decline in effect after reaching peak blood concentrations. The clinical correlation of this was found in comparing math problems attempted and solved between a mixed amphetamine salts preparation (MAS) 10 mg once at 8 am vs 8 am followed by 12 pm [14]. The study also demonstrated the phenomenon of acute tolerance, where even if blood concentrations were maintained over the course of the day, clinical efficacy in the form of math problems attempted and solved would diminish over the course of the day. These findings eventually led to the development of a once daily preparation (MAS XR) [15], which is a composition of 50% immediate-release beads and 50% delayed release beads intended to mimic this twice-daily dosing with only a single administration.
- ^ a b Swanson JM, Volkow ND (January 2009). "Psychopharmacology: concepts and opinions about the use of stimulant medications". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 50 (1–2): 180–193. doi:10.1111/j.1469-7610.2008.02062.x. PMC 2681087. PMID 19220601.
- ^ Dolder PC, Strajhar P, Vizeli P, Hammann F, Odermatt A, Liechti ME (2017). "Pharmacokinetics and Pharmacodynamics of Lisdexamfetamine Compared with D-Amphetamine in Healthy Subjects". Frontiers in Pharmacology. 8: 617. doi:10.3389/fphar.2017.00617. PMC 5594082. PMID 28936175.
- ^ Folgering JH, Choi M, Schlumbohm C, van Gaalen MM, Stratford RE (April 2019). "Development of a non-human primate model to support CNS translational research: Demonstration with D-amphetamine exposure and dopamine response". Journal of Neuroscience Methods. 317: 71–81. doi:10.1016/j.jneumeth.2019.02.005. PMID 30768951.
- ^ van Gaalen MM, Schlumbohm C, Folgering JH, Adhikari S, Bhattacharya C, Steinbach D, Stratford RE (April 2019). "Development of a Semimechanistic Pharmacokinetic-Pharmacodynamic Model Describing Dextroamphetamine Exposure and Striatal Dopamine Response in Rats and Nonhuman Primates following a Single Dose of Dextroamphetamine". The Journal of Pharmacology and Experimental Therapeutics. 369 (1): 107–120. doi:10.1124/jpet.118.254508. PMID 30733244.
- ^ Baumeister AA (2021). "Is Attention-Deficit/Hyperactivity Disorder a Risk Syndrome for Parkinson's Disease?". Harvard Review of Psychiatry. 29 (2): 142–158. doi:10.1097/HRP.0000000000000283. PMID 33560690.
It has been suggested that the association between PD and ADHD may be explained, in part, by toxic effects of these drugs on DA neurons.241 [...] An important question is whether amphetamines, as they are used clinically to treat ADHD, are toxic to DA neurons. In most of the animal and human studies cited above, stimulant exposure levels are high relative to clinical doses, and dosing regimens (as stimulants) rarely mimic the manner in which these drugs are used clinically. The study by Ricaurte and colleagues248 is an exception. In that study, baboons orally self-administered a racemic (3:1 d/l) amphetamine mixture twice daily in increasing doses ranging from 2.5 to 20 mg/day for four weeks. Plasma amphetamine concentrations, measured at one-week intervals, were comparable to those observed in children taking amphetamine for ADHD. Two to four weeks after cessation of amphetamine treatment, multiple markers of striatal DA function were decreased, including DA and DAT. In another group of animals (squirrel monkeys), d/l amphetamine blood concentration was titrated to clinically comparable levels for four weeks by administering varying doses of amphetamine by orogastric gavage. These animals also had decreased markers of striatal DA function assessed two weeks after cessation of amphetamine.
- ^ Advokat C (July 2007). "Update on amphetamine neurotoxicity and its relevance to the treatment of ADHD". Journal of Attention Disorders. 11 (1): 8–16. doi:10.1177/1087054706295605. PMID 17606768.
Recently, however, new data from Ricaurte et al. (2005) indicate that primates may be much more susceptible than rats to AMPH-induced neurotoxicity. They examined the effect of the drug in adult baboons and squirrel monkeys, as clinically used to treat ADHD. In the first two studies, baboons were trained to orally selfadminister a mixture of AMPH salts (a 3:1 ratio of dextro [S(+)] and levo [R(-)] AMPH, which simulated a common formulation for ADHD treatment). AMPH was administered twice daily for approximately 4 weeks at escalating doses of 2.5 to 20 mg (0.67 to 1.00 mg/kg). During the second study, plasma AMPH concentrations were determined at the end of each week. In the third study, AMPH was administered by orogastric gavage to squirrel monkeys and doses were adjusted (to 0.58-0.68 mg/kg) so that for approximately the last 3 weeks plasma drug concentrations were comparable to those reported in clinical populations of children receiving chronic AMPH treatment—100 to 150 ng/ml (McGough et al., 2003). Measurements in all three investigations were taken 2 to 4 weeks after drug treatment. Results from the first two studies showed significant reductions in striatal dopamine concentration, dopamine transporter density, and vesicular monoamine transporter sites. Plasma AMPH concentration at the end of the 4 week treatment period was 168 ± 25 ng/ml. In squirrel monkeys, brain dopamine concentrations and vesicular transporter sites were also significantly reduced although dopamine transporter decreases were not statistically significant. These results raise obvious concerns about clinical drug treatment of ADHD, although extrapolation to human populations may be premature until possible species differences in mechanism of action, developmental variables, or metabolism are determined.
- ^ Asser A, Taba P (2015). "Psychostimulants and movement disorders". Frontiers in Neurology. 6: 75. doi:10.3389/fneur.2015.00075. PMC 4403511. PMID 25941511.
Amphetamine treatment similar to that used for ADHD has been demonstrated to produce brain dopaminergic neurotoxicity in primates, causing the damage of dopaminergic nerve endings in the striatum that may also occur in other disorders with long-term amphetamine treatment (57).
- ^ Courtney KE, Ray LA (2016). "Clinical neuroscience of amphetamine-type stimulants". Clinical neuroscience of amphetamine-type stimulants: From basic science to treatment development. Progress in Brain Research. Vol. 223. pp. 295–310. doi:10.1016/bs.pbr.2015.07.010. ISBN 978-0-444-63545-7. PMID 26806782.
Repeated exposure to moderate to high levels of methamphetamine has been related to neurotoxic effects on the dopaminergic and serotonergic systems, leading to potentially irreversible loss of nerve terminals and/or neuron cell bodies (Cho and Melega, 2002). Preclinical evidence suggests that d-amphetamine, even when administered at commonly prescribed therapeutic doses, also results in toxicity to brain dopaminergic axon terminals (Ricaurte et al., 2005).
- ^ Berman SM, Kuczenski R, McCracken JT, London ED (February 2009). "Potential adverse effects of amphetamine treatment on brain and behavior: a review". Molecular Psychiatry. 14 (2): 123–142. doi:10.1038/mp.2008.90. PMC 2670101. PMID 18698321.
Though the paradigm used by Ricaurte et al. 53 arguably still incorporates amphetamine exposure at a level above much clinical use,14,55 it raises important unanswered questions. Is there a threshold of amphetamine exposure above which persistent changes in the dopamine system are induced? [...]
- ^ Ricaurte GA, Mechan AO, Yuan J, Hatzidimitriou G, Xie T, Mayne AH, McCann UD (October 2005). "Amphetamine treatment similar to that used in the treatment of adult attention-deficit/hyperactivity disorder damages dopaminergic nerve endings in the striatum of adult nonhuman primates". The Journal of Pharmacology and Experimental Therapeutics. 315 (1): 91–98. doi:10.1124/jpet.105.087916. PMID 16014752.
- ^ Hart XM, Spangemacher M, Defert J, Uchida H, Gründer G (April 2024). "Update Lessons from PET Imaging Part II: A Systematic Critical Review on Therapeutic Plasma Concentrations of Antidepressants". Ther Drug Monit. 46 (2): 155–169. doi:10.1097/FTD.0000000000001142. PMID 38287888.
- ^ Eap CB, Gründer G, Baumann P, Ansermot N, Conca A, Corruble E, Crettol S, Dahl ML, de Leon J, Greiner C, Howes O, Kim E, Lanzenberger R, Meyer JH, Moessner R, Mulder H, Müller DJ, Reis M, Riederer P, Ruhe HG, Spigset O, Spina E, Stegman B, Steimer W, Stingl J, Suzen S, Uchida H, Unterecker S, Vandenberghe F, Hiemke C (October 2021). "Tools for optimising pharmacotherapy in psychiatry (therapeutic drug monitoring, molecular brain imaging and pharmacogenetic tests): focus on antidepressants" (PDF). The World Journal of Biological Psychiatry. 22 (8): 561–628. doi:10.1080/15622975.2021.1878427. PMID 33977870. S2CID 234472488. Archived (PDF) from the original on 5 May 2022. Retrieved 10 April 2022.
- ^ Carroll FI, Blough BE, Mascarella SW, Navarro HA, Lukas RJ, Damaj MI (2014). "Bupropion and bupropion analogs as treatments for CNS disorders". Emerging Targets & Therapeutics in the Treatment of Psychostimulant Abuse. Advances in Pharmacology. Vol. 69. Academic Press. pp. 177–216. doi:10.1016/B978-0-12-420118-7.00005-6. ISBN 978-0-12-420118-7. PMID 24484978.
- ^ Verbeeck W, Bekkering GE, Van den Noortgate W, Kramers C (October 2017). "Bupropion for attention deficit hyperactivity disorder (ADHD) in adults". The Cochrane Database of Systematic Reviews. 2017 (10): CD009504. doi:10.1002/14651858.CD009504.pub2. PMC 6485546. PMID 28965364.
- ^ Jenner P, Mori A, Kanda T (November 2020). "Can adenosine A2A receptor antagonists be used to treat cognitive impairment, depression or excessive sleepiness in Parkinson's disease?". Parkinsonism Relat Disord. 80 Suppl 1: S28–S36. doi:10.1016/j.parkreldis.2020.09.022. PMID 33349577.
- ^ a b Turner V, Husain M (2022). "Anhedonia in Neurodegenerative Diseases". Curr Top Behav Neurosci. Current Topics in Behavioral Neurosciences. 58: 255–277. doi:10.1007/7854_2022_352. ISBN 978-3-031-09682-2. PMID 35435648.
Recently, PD patients have been treated with istradefylline, an adenosine A2A receptor antagonist used for treatment of motor symptoms. The drug was given to 14 PD patients for 12 weeks, measuring anhedonia, apathy and depression using the SHAPS, Apathy Scale and BDI. On istradefylline, SHAPS, Apathy Scale and BDI scores significantly reduced from baseline scores at 4-, 8- and 12-weeks, with mean SHAPS scores at week 12 about 50% reduced from baseline scores, indicating that istradefylline reduces anhedonia (Nagayama et al. 2019). As apathy and depression rates dropped as well as anhedonia, this trial also provided evidence for the overlapping relationship between the three symptoms. [...] Taken together, there is some evidence that dopamine agonists such as pramipexole and piribedil, or the adenosine A2A receptor antagonist istradefylline can improve anhedonia and apathy in PD.
- ^ Ribeiro JA, Sebastião AM (2010). "Caffeine and adenosine". J Alzheimers Dis. 20 Suppl 1: S3–15. doi:10.3233/JAD-2010-1379. hdl:10451/6361. PMID 20164566.
- ^ Jamwal S, Mittal A, Kumar P, Alhayani DM, Al-Aboudi A (2019). "Therapeutic Potential of Agonists and Antagonists of A1, A2a, A2b and A3 Adenosine Receptors". Curr Pharm Des. 25 (26): 2892–2905. doi:10.2174/1381612825666190716112319. PMID 31333104.
- ^ Froestl W, Muhs A, Pfeifer A (2012). "Cognitive enhancers (nootropics). Part 1: drugs interacting with receptors" (PDF). Journal of Alzheimer's Disease. 32 (4): 793–887. doi:10.3233/JAD-2012-121186. PMID 22886028. S2CID 10511507. Archived from the original (PDF) on 15 November 2020.
- ^ Nunes EJ, Randall PA, Santerre JL, Given AB, Sager TN, Correa M, Salamone JD (September 2010). "Differential effects of selective adenosine antagonists on the effort-related impairments induced by dopamine D1 and D2 antagonism". Neuroscience. 170 (1): 268–280. doi:10.1016/j.neuroscience.2010.05.068. PMC 3268040. PMID 20600675.
- ^ a b c Nunes EJ, Randall PA, Podurgiel S, Correa M, Salamone JD (November 2013). "Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: effects of drugs acting on dopamine, adenosine, and muscarinic acetylcholine receptors". Neurosci Biobehav Rev. 37 (9 Pt A): 2015–2025. doi:10.1016/j.neubiorev.2013.04.002. PMID 23583616.
- ^ Pardo M, Lopez-Cruz L, Valverde O, Ledent C, Baqi Y, Müller CE, Salamone JD, Correa M (April 2012). "Adenosine A2A receptor antagonism and genetic deletion attenuate the effects of dopamine D2 antagonism on effort-based decision making in mice". Neuropharmacology. 62 (5–6): 2068–2077. doi:10.1016/j.neuropharm.2011.12.033. PMID 22261384.
- ^ a b Vecchio I, Sorrentino L, Paoletti A, Marra R, Arbitrio M (2021). "The State of The Art on Acetylcholinesterase Inhibitors in the Treatment of Alzheimer's Disease". J Cent Nerv Syst Dis. 13: 11795735211029113. doi:10.1177/11795735211029113. PMC 8267037. PMID 34285627.
- ^ Krall WJ, Sramek JJ, Cutler NR (April 1999). "Cholinesterase inhibitors: a therapeutic strategy for Alzheimer disease". Ann Pharmacother. 33 (4): 441–450. doi:10.1345/aph.18211. PMID 10332536.
- ^ Birks J (January 2006). "Cholinesterase inhibitors for Alzheimer's disease". Cochrane Database Syst Rev. 2006 (1): CD005593. doi:10.1002/14651858.CD005593. PMC 9006343. PMID 16437532.
- ^ Mirza NR, Peters D, Sparks RG (2003). "Xanomeline and the antipsychotic potential of muscarinic receptor subtype selective agonists". CNS Drug Rev. 9 (2): 159–186. doi:10.1111/j.1527-3458.2003.tb00247.x. PMC 6741650. PMID 12847557.
- ^ Paul SM, Yohn SE, Brannan SK, Neugebauer NM, Breier A (October 2024). "Muscarinic Receptor Activators as Novel Treatments for Schizophrenia". Biol Psychiatry. 96 (8): 627–637. doi:10.1016/j.biopsych.2024.03.014. PMID 38537670.
- ^ "Cobenfy (xanomeline and trospium chloride) capsules, for oral use" (PDF). Bristol-Myers Squibb.
- ^ a b Azhar L, Kusumo RW, Marotta G, Lanctôt KL, Herrmann N (February 2022). "Pharmacological Management of Apathy in Dementia". CNS Drugs. 36 (2): 143–165. doi:10.1007/s40263-021-00883-0. PMID 35006557.
- ^ Rodda J, Morgan S, Walker Z (October 2009). "Are cholinesterase inhibitors effective in the management of the behavioral and psychological symptoms of dementia in Alzheimer's disease? A systematic review of randomized, placebo-controlled trials of donepezil, rivastigmine and galantamine". Int Psychogeriatr. 21 (5): 813–824. doi:10.1017/S1041610209990354 (inactive 1 November 2024). PMID 19538824.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link) - ^ Reilly S, Dhaliwal S, Arshad U, Macerollo A, Husain N, Costa AD (February 2024). "The effects of rivastigmine on neuropsychiatric symptoms in the early stages of Parkinson's disease: A systematic review". Eur J Neurol. 31 (2): e16142. doi:10.1111/ene.16142. PMC 11236000. PMID 37975761.
- ^ Fasipe, OlumuyiwaJohn (2018). "Neuropharmacological classification of antidepressant agents based on their mechanisms of action". Archives of Medicine and Health Sciences. 6 (1). Medknow: 81. doi:10.4103/amhs.amhs_7_18. ISSN 2321-4848.
- ^ a b Thome J, Foley P (August 2015). "Agomelatine: an agent against anhedonia and abulia?". J Neural Transm (Vienna). 122 Suppl 1: S3–S7. doi:10.1007/s00702-013-1126-6. PMID 24311062.
- ^ Harrison F, Aerts L, Brodaty H (November 2016). "Apathy in Dementia: Systematic Review of Recent Evidence on Pharmacological Treatments". Curr Psychiatry Rep. 18 (11): 103. doi:10.1007/s11920-016-0737-7. PMID 27726067.
- ^ Theleritis C, Siarkos K, Politis A, Smyrnis N, Papageorgiou C, Politis AM (July 2023). "A Systematic Review of Pharmacological Interventions for Apathy in Aging Neurocognitive Disorders". Brain Sci. 13 (7): 1061. doi:10.3390/brainsci13071061. PMC 10377475. PMID 37508993.
- ^ Callegari I, Mattei C, Benassi F, Krueger F, Grafman J, Yaldizli Ö, Sassos D, Massucco D, Scialò C, Nobili F, Serrati C, Amore M, Cocito L, Emberti Gialloreti L, Pardini M (2016). "Agomelatine Improves Apathy in Frontotemporal Dementia". Neurodegener Dis. 16 (5–6): 352–356. doi:10.1159/000445873. PMID 27229348.
- ^ De Berardis D, Valchera A, Fornaro M, Serroni N, Marini S, Moschetta FS, Martinotti G, Di Giannantonio M (April 2013). "Agomelatine reversal of escitalopram-induced apathy: a case report". Psychiatry Clin Neurosci. 67 (3): 190–191. doi:10.1111/pcn.12032. PMID 23581873.
- ^ a b Zhang R, Chen J (December 2023). "Research progress on the role of orphan receptor GPR139 in neuropsychiatric behaviours". Eur J Pharmacol. 960: 176150. doi:10.1016/j.ejphar.2023.176150. PMID 38059447.
In 2021, Reichard et al., (2021) developed the GPR139 agonist TAK041, also known as NBI-1065846. TAK-041 has good physical and chemical properties, can cross the blood–brain barrier, and shows potential in preclinical studies to treat schizophrenia symptoms. Several clinical trials indicate that TAK-041 is safe and metabolically stable (Kamel et al., 2021; Reichard et al., 2021; Yin et al., 2022). Apathy is a condition characterised by a lack of motivation, emotion, or interest and is a common symptom of many psychiatric and neurological disorders. Munster et al. (2022) provided preclinical evidence supporting GPR139 agonism (using TAK-041) as a molecular mechanism for treating apathy. The research and development of TAK-041 have effectively promoted the process of de-orphaning GPR139 and its clinical application value.
- ^ a b Münster A, Sommer S, Kúkeľová D, Sigrist H, Koros E, Deiana S, Klinder K, Baader-Pagler T, Mayer-Wrangowski S, Ferger B, Bretschneider T, Pryce CR, Hauber W, von Heimendahl M (August 2022). "Effects of GPR139 agonism on effort expenditure for food reward in rodent models: Evidence for pro-motivational actions". Neuropharmacology. 213: 109078. doi:10.1016/j.neuropharm.2022.109078. PMID 35561791.
- ^ a b "TAK 041". AdisInsight. 26 September 2023. Retrieved 26 September 2024.
- ^ Lu Y, Hatzipantelis CJ, Langmead CJ, Stewart GD (July 2024). "Molecular insights into orphan G protein-coupled receptors relevant to schizophrenia". Br J Pharmacol. 181 (14): 2095–2113. doi:10.1111/bph.16221. PMID 37605621.
- ^ Reichard HA, Schiffer HH, Monenschein H, Atienza JM, Corbett G, Skaggs AW, Collia DR, Ray WJ, Serrats J, Bliesath J, Kaushal N, Lam BP, Amador-Arjona A, Rahbaek L, McConn DJ, Mulligan VJ, Brice N, Gaskin PL, Cilia J, Hitchcock S (August 2021). "Discovery of TAK-041: a Potent and Selective GPR139 Agonist Explored for the Treatment of Negative Symptoms Associated with Schizophrenia". J Med Chem. 64 (15): 11527–11542. doi:10.1021/acs.jmedchem.1c00820. PMID 34260228.
- ^ "Neurocrine Biosciences Provides Development Pipeline Update". Neurocrine Biosciences. 9 November 2023. Retrieved 26 September 2024.
The investigational NBI-1065846, as part of the collaboration with Takeda Pharmaceutical Company Limited (Takeda), did not meet its primary endpoint in the Phase 2 TERPSIS™ study evaluating its efficacy compared to placebo in patients with anhedonia in major depressive disorder. No further development with NBI-1065846 is planned at this time.
- ^ a b Lee Y, Subramaniapillai M, Brietzke E, Mansur RB, Ho RC, Yim SJ, McIntyre RS (December 2018). "Anti-cytokine agents for anhedonia: targeting inflammation and the immune system to treat dimensional disturbances in depression". Ther Adv Psychopharmacol. 8 (12): 337–348. doi:10.1177/2045125318791944. PMC 6278744. PMID 30524702.
- ^ Treadway MT, Etuk SM, Cooper JA, Hossein S, Hahn E, Betters SA, Liu S, Arulpragasam AR, DeVries BA, Irfan N, Nuutinen MR, Wommack EC, Woolwine BJ, Bekhbat M, Kragel PA, Felger JC, Haroon E, Miller AH (September 2024). "A randomized proof-of-mechanism trial of TNF antagonism for motivational deficits and related corticostriatal circuitry in depressed patients with high inflammation". Mol Psychiatry. doi:10.1038/s41380-024-02751-x. PMID 39289477.
- ^ Rahmati-Dehkordi F, Birang N, Jalalian MN, Tamtaji Z, Dadgostar E, Aschner M, Shafiee Ardestani M, Jafarpour H, Mirzaei H, Nabavizadeh F, Tamtaji OR (September 2024). "Can infliximab serve as a new therapy for neuropsychiatric symptoms?". Naunyn Schmiedebergs Arch Pharmacol. doi:10.1007/s00210-024-03397-w. PMID 39225829.
- ^ Rizk MM, Bolton L, Cathomas F, He H, Russo SJ, Guttman-Yassky E, Mann JJ, Murrough J (June 2024). "Immune-Targeted Therapies for Depression: Current Evidence for Antidepressant Effects of Monoclonal Antibodies". J Clin Psychiatry. 85 (3). doi:10.4088/JCP.23nr15243. PMID 38959503.
- ^ Tay J, Morris RG, Markus HS (July 2021). "Apathy after stroke: Diagnosis, mechanisms, consequences, and treatment". Int J Stroke. 16 (5): 510–518. doi:10.1177/1747493021990906. PMC 8267086. PMID 33527880.
- ^ a b Yohn SE, Collins SL, Contreras-Mora HM, Errante EL, Rowland MA, Correa M, Salamone JD (February 2016). "Not All Antidepressants Are Created Equal: Differential Effects of Monoamine Uptake Inhibitors on Effort-Related Choice Behavior". Neuropsychopharmacology. 41 (3): 686–694. doi:10.1038/npp.2015.188. PMC 4707815. PMID 26105139.
- ^ Yohn SE, Errante EE, Rosenbloom-Snow A, Somerville M, Rowland M, Tokarski K, Zafar N, Correa M, Salamone JD (October 2016). "Blockade of uptake for dopamine, but not norepinephrine or 5-HT, increases selection of high effort instrumental activity: Implications for treatment of effort-related motivational symptoms in psychopathology". Neuropharmacology. 109: 270–280. doi:10.1016/j.neuropharm.2016.06.018. PMID 27329556.
- ^ Camino S, Strejilevich SA, Godoy A, Smith J, Szmulewicz A (July 2023). "Are all antidepressants the same? The consumer has a point". Psychological Medicine. 53 (9): 4004–4011. doi:10.1017/S0033291722000678. PMID 35346413.
- ^ Harsing LG, Timar J, Miklya I (August 2023). "Striking Neurochemical and Behavioral Differences in the Mode of Action of Selegiline and Rasagiline". Int J Mol Sci. 24 (17): 13334. doi:10.3390/ijms241713334. PMC 10487936. PMID 37686140.
- ^ Miklya I (June 2014). "Essential difference between the pharmacological spectrum of (-)-deprenyl and rasagiline". Pharmacol Rep. 66 (3): 453–458. doi:10.1016/j.pharep.2013.11.003. PMID 24905523.
- ^ Lieberman JA (2004). "Dopamine partial agonists: a new class of antipsychotic". CNS Drugs. 18 (4): 251–267. doi:10.2165/00023210-200418040-00005. PMID 15015905.
- ^ a b Taylor D, Chithiramohan R, Grewal J, Gupta A, Hansen L, Reynolds GP, Pappa S (September 2023). "Dopamine partial agonists: a discrete class of antipsychotics". Int J Psychiatry Clin Pract. 27 (3): 272–284. doi:10.1080/13651501.2022.2151473. PMID 36495086.
- ^ de Bartolomeis A, Barone A, Begni V, Riva MA (February 2022). "Present and future antipsychotic drugs: A systematic review of the putative mechanisms of action for efficacy and a critical appraisal under a translational perspective". Pharmacol Res. 176: 106078. doi:10.1016/j.phrs.2022.106078. hdl:2434/909466. PMID 35026403.
Aripiprazole, brexpiprazole, and cariprazine are representative of a new class of APs that act as "dopamine stabilizers", namely partial agonists at D2R/D3R [16]. Partial agonists may act as functional agonists or antagonists, depending on the surrounding levels of endogenous ligand. According to this view, D2R partial agonists may act as functional antagonists within the mesolimbic system, where a hyperdopaminergic state may contribute to positive symptoms; on the other hand, they act as functional agonists in the mesocortical pathway, where extracellular dopamine levels are low, thus mitigating, or at least not worsening, negative and cognitive symptoms [17], [18]. [...]
- ^ Bolstad I, Andreassen OA, Groote I, Server A, Sjaastad I, Kapur S, Jensen J (December 2015). "Effects of haloperidol and aripiprazole on the human mesolimbic motivational system: A pharmacological fMRI study". Eur Neuropsychopharmacol. 25 (12): 2252–2261. doi:10.1016/j.euroneuro.2015.09.016. hdl:10852/50193. PMID 26476705.
Accordingly, the task-related BOLD-fMRI response in the mesolimbic motivational system was diminished in the haloperidol group compared to the placebo group, particularly in the ventral striatum, whereas the aripiprazole group showed task-related activations intermediate of the placebo and haloperidol groups.
- ^ Scheggi S, De Montis MG, Gambarana C (November 2018). "Making Sense of Rodent Models of Anhedonia". Int J Neuropsychopharmacol. 21 (11): 1049–1065. doi:10.1093/ijnp/pyy083. PMC 6209858. PMID 30239762.
In self-administration protocols, the schedule used to assess the motivation to work for a natural (or a drug) reward is commonly the progressive ratio (PR) schedule (Hodos, 1961) where increasing effort is required to obtain the reward as the ratio requirement progressively increases, and the last ratio completed is the breaking point. The breaking point measures the effort the animal is willing to exert to obtain the reinforcing stimulus and is then considered an index of motivation, or of the perceived reinforcing value of the stimulus. Thus, a decrease in breaking point may be regarded as a core symptom in animal models of anhedonia, although this decrease is not reliably observed in all the models. Reductions in breakpoints for sucrose have been reported in a genetic animal model of depression, the congenital learned helpless rat (Vollmayr et al., 2004), in a chronic unavoidable stress protocol in rats (Marchese et al., 2013; Scheggi et al., 2016), and in rats and mice exposed to chronic social defeat (Bergamini et al., 2016; Spierling et al., 2017). This index of reduced motivation for a natural reward can be restored to control values by treatments endowed with antidepressant and/or promotivational activity, for example, lithium, clozapine, aripiprazole, and lamotrigine (Marchese et al., 2013; Scheggi et al., 2015, 2017b; Scheggi, Pelliccia, De Montis and Gambarana, unpublished data). Conversely, exposure to the CMS model does not usually affect sucrose breaking point.
- ^ Scheggi S, Pelliccia T, Gambarana C, De Montis MG (February 2018). "Aripiprazole relieves motivational anhedonia in rats". J Affect Disord. 227: 192–197. doi:10.1016/j.jad.2017.10.032. PMID 29100151.
- ^ Keks N, Hope J, Schwartz D, McLennan H, Copolov D, Meadows G (May 2020). "Comparative Tolerability of Dopamine D2/3 Receptor Partial Agonists for Schizophrenia". CNS Drugs. 34 (5): 473–507. doi:10.1007/s40263-020-00718-4. PMID 32246399.
- ^ a b Ecevitoglu A, Meka N, Rotolo RA, Edelstein GA, Srinath S, Beard KR, Carratala-Ros C, Presby RE, Cao J, Okorom A, Newman AH, Correa M, Salamone JD (July 2024). "Potential therapeutics for effort-related motivational dysfunction: assessing novel atypical dopamine transport inhibitors". Neuropsychopharmacology. 49 (8): 1309–1317. doi:10.1038/s41386-024-01826-1. PMC 11224370. PMID 38429498.