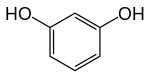
Resorcinol (or resorcin) is a phenolic compound. It is an organic compound with the formula C6H4(OH)2. It is one of three isomeric benzenediols, the 1,3-isomer (or meta-isomer). Resorcinol crystallizes from benzene as colorless needles that are readily soluble in water, alcohol, and ether, but insoluble in chloroform and carbon disulfide.[6]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Benzene-1,3-diol[1] | |||
| Other names
Resorcinol[1]
Resorcin m-Dihydroxybenzene 1,3-Benzenediol 1,3-Dihydroxybenzene 3-Hydroxyphenol m-Benzenediol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.260 | ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| UN number | 2876 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H6O2 | |||
| Molar mass | 110.111 g/mol | ||
| Appearance | White solid[2] | ||
| Odor | Faint[2] | ||
| Density | 1.28 g/cm3, solid | ||
| Melting point | 110 °C (230 °F; 383 K) | ||
| Boiling point | 277 °C (531 °F; 550 K) | ||
| 110 g/100 mL at 20 °C | |||
| Vapor pressure | 0.0002 mmHg (25 °C)[2] | ||
| Acidity (pKa) | 9.15[3] | ||
| −67.26×10−6 cm3/mol | |||
Refractive index (nD)
|
1.578[4] | ||
| 2.07±0.02 D[5] | |||
| Thermochemistry | |||
Std enthalpy of
formation (ΔfH⦵298) |
-368.0 kJ·mol−1[4] | ||
Enthalpy of fusion (ΔfH⦵fus)
|
20.4 kJ·mol−1[4] | ||
| Pharmacology | |||
| D10AX02 (WHO) S01AX06 (WHO) | |||
| Hazards | |||
| GHS labelling: | |||
  
| |||
| H302, H313, H315, H318, H400 | |||
| P273, P280, P305+P351+P338 | |||
| Flash point | 127 °C; 261 °F; 400 K[2] | ||
| 608 °C (1,126 °F; 881 K)[4] | |||
| Explosive limits | 1.4%-?[2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
none[2] | ||
REL (Recommended)
|
TWA 10 ppm (45 mg/m3) ST 20 ppm (90 mg/m3)[2] | ||
IDLH (Immediate danger)
|
N.D.[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Production
editResorcinol is produced in several steps from benzene, starting with dialkylation with propylene to give 1,3-diisopropylbenzene. Oxidation and Hock rearrangement of this disubstituted arene gives acetone and resorcinol.[6]
Resorcinol is an expensive chemical, produced in only a very few locations around the world (as of 2010 only four commercial plants were known to be operative: in the United States, Germany, China, and Japan), and as such it is the determining factor in the cost of PRF adhesives.[7] Production in the United States ended in 2017 with the closure of Indspec Chemical's plant in Petrolia, Pennsylvania.[8]
Many additional routes exist for resorcinol. It was formerly produced by disulfonation of benzene followed by hydrolysis of the 1,3-disulfonate. This method has been discarded because it cogenerates so much sulfur-containing waste. Resorcinol can also be produced when any of a large number of resins (such as galbanum and asafoetida) are melted with potassium hydroxide, or by the distillation of Brazilwood extract. It may be synthesized by melting 3-iodophenol, phenol-3-sulfonic acid with potassium carbonate. Diazotization of 3-aminophenol or on 1,3-diaminobenzene followed by hydrolysis provides yet another route.[9] Many ortho- and para-compounds of the aromatic series (for example, the bromophenols, benzene-para-disulfonic acid) also yield resorcinol on fusion with potassium hydroxide.
Reactions
editPartial hydrogenation of resorcinol gives dihydroresorcinol, also known as 1,3-cyclohexanedione.[10][11]
It reduces Fehling's solution and ammoniacal silver solutions. It does not form a precipitate with lead acetate solution, as does the isomeric pyrocatechol. Iron(III) chloride colors its aqueous solution a dark-violet, and bromine water precipitates tribromoresorcinol. These properties are what give it its use as a colouring agent for certain chromatography experiments.
Sodium amalgam reduces it to dihydroresorcin, which when heated to 150 to 160 °C with concentrated barium hydroxide solution gives γ-acetylbutyric acid.[citation needed]
When fused with potassium hydroxide, resorcinol yields phloroglucin, pyrocatechol, and diresorcinol. It condenses with acids or acid chlorides, in the presence of dehydrating agents, to oxyketones, for example, with zinc chloride and glacial acetic acid at 145 °C it yields resacetophenone (HO)2C6H3COCH3.[12] With the anhydrides of dibasic acids, it yields fluoresceins. When heated with calcium chloride—ammonia to 200 °C it yields meta-dioxydiphenylamine.[13]
With sodium nitrite it forms a water-soluble blue dye, which is turned red by acids, and is used as a pH indicator under the name of lacmoid.[14] It condenses readily with aldehydes, yielding with formaldehyde, on the addition of catalytic hydrochloric acid, methylene diresorcin [(HO)C6H3(O)]2CH2. Reaction with chloral hydrate in the presence of potassium bisulfate yields the lactone of tetra-oxydiphenyl methane carboxylic acid.[15] In alcoholic solution it condenses with sodium acetoacetate to form 4-methylumbelliferone.[16]
In presence of Sulfuric acid, with twice amount of Succinic acid, Resorcinol creates Fluorescence effect on water.[17]
In addition to electrophilic aromatic addition, resorcinol (and other polyols) undergo nucleophilic substitution via the enone tautomer.
Nitration with concentrated nitric acid in the presence of cold concentrated sulfuric acid yields trinitroresorcin (styphnic acid), an explosive.
Occurrence and use
editDerivatives of resorcinol are found in different natural sources. Alkylresorcinols are found in rye.[18] Polyresorcinols are found as pseudotannins in plants.[17]
Adhesives
editResorcinol is mainly used in the production of resins. As a mixture with phenol, it condenses with formaldehyde to afford adhesives. Such resins are used as adhesives in the rubber industry and others are used for wood glue.[6] In relation to its conversion resins with formaldehyde, resorcinol is the starting material for resorcinarene rings.
Medical uses
editIt is present in over-the-counter topical acne treatments at 2% or less concentration, and in prescription treatments at higher concentrations.[19] Monoacetylresorcinol, C6H4(OH)(O–COCH3), is used under the name of Euresol.[20] It is used in hidradenitis suppurativa with limited evidence showing it can help with resolution of the lesions.[21] Resorcinol is one of the active ingredients in products such as Resinol, Vagisil, and Clearasil.
In the 1950s and early 1960s the British Army used it, in the form of a paste applied directly to the skin. One such place where this treatment was given to soldiers with chronic acne was the Cambridge Military Hospital, Aldershot, England. It was not always successful.
4-Hexylresorcinol is an anesthetic found in throat lozenges.
Chemical uses
editResorcinol is used as a chemical intermediate for the synthesis of pharmaceuticals and other organic compounds. It is used in the production of diazo dyes and plasticizers and as a UV absorber in resins.
It is an analytical reagent for the qualitative determination of ketoses (Seliwanoff's test).
It is the starting material for the initiating explosive lead styphnate.[22]
Related compounds
editResazurin, C12H7NO4, obtained by the action of nitrous acid on resorcinol,[23] forms small dark red crystals possessing a greenish metallic glance. When dissolved in concentrated sulfuric acid and warmed to 210 °C, the solution on pouring into water yields a precipitate of resorufin, C12H7NO3, an oxyphenoxazone, which is insoluble in water but is readily soluble in hot concentrated hydrochloric acid, and in solutions of caustic alkalis. The alkaline solutions are of a rose-red color and show a cinnabar-red fluorescence. A tetrabromresorufin is used as a dyestuff under the name of Fluorescent Resorcin Blue.
Thioresorcinol is obtained by the action of zinc and hydrochloric acid on meta-benzenedisulfonyl chloride. It melts at 27 °C and boils at 243 °C. Resorcinol disulfonic acid, (HO)2C6H2(HSO3)2, is a deliquescent mass obtained by the action of sulfuric acid on resorcin.[24] It is readily soluble in water and ethanol.
Resorcinol is also a common scaffold that is found in a class of anticancer agents, some of which (luminespib, ganetespib, KW-2478, and onalespib) were in clinical trials as of 2014[update].[25][26] Part of the resorcinol structure binds to inhibits the N-terminal domain of heat shock protein 90, which is a drug target for anticancer treatments.[25]
History, etymology, and nomenclature
editAustrian chemist Heinrich Hlasiwetz (1825–1875) is remembered for his chemical analysis of resorcinol and for his part in the first preparation of resorcinol, along with Ludwig Barth, which was published in 1864.[27]: 10 [28]
Benzene-1,3-diol is the name recommended by the International Union of Pure and Applied Chemistry (IUPAC) in its 1993 Recommendations for the Nomenclature of Organic Chemistry.[29]
Resorcinol is so named because of its derivation from ammoniated resin gum, and for its relation to the chemical orcinol.[30]
Toxicity
editResorcinol has low toxicity, with an LD50 (rats, oral) > 300 mg/kg. It is less toxic than phenol.[6]
Resorcinol was named a substance of very high concern under European Union REACH in 2022 because of its endocrine disrupting properties.[31]
References
edit- ^ a b "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 691. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ a b c d e f g h NIOSH Pocket Guide to Chemical Hazards. "#0543". National Institute for Occupational Safety and Health (NIOSH).
- ^ Gawron, O.; Duggan, M.; Grelechi, C. (1952). "Manometric Determination of Dissociation Constants of Phenols". Analytical Chemistry. 24 (6): 969–970. doi:10.1021/ac60066a013.
- ^ a b c d CRC handbook of chemistry and physics: a ready-reference book of chemical and physical data. William M. Haynes, David R. Lide, Thomas J. Bruno (2016-2017, 97th ed.). Boca Raton, Florida. 2016. ISBN 978-1-4987-5428-6. OCLC 930681942.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ Lander, John J.; Svirbely, John J. Lander, W. J. (1945). "The Dipole Moments of Catechol, Resorcinol and Hydroquinone". Journal of the American Chemical Society. 67 (2): 322–324. doi:10.1021/ja01218a051.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d K. W. Schmiedel; D. Decker (2012). "Resorcinol". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a23_111.pub2. ISBN 978-3527306732.
- ^ Wood adhesives, Pizzi & Mittal, 2010
- ^ McCoy, Michael. "Resorcinol plant to close". Chemical & Engineering News. American Chemical Society. Retrieved 6 December 2023.
- ^ Meyer, J (1897). "Notiz über die Umwandlung von Aminen in Phenole". Berichte der Deutschen Chemischen Gesellschaft. 30 (3): 2568–2569. doi:10.1002/cber.18970300334.
- ^ Thompson, R. B. (1947). "Dihydroresorcinol". Org. Synth. 27: 21. doi:10.15227/orgsyn.027.0021.
- ^ Mekler, A. B.; Ramachandran, S.; Swaminathan, S.; Newman, Melvin S. (1961). "Methyl-1,3-Cyclohexanedione". Org. Synth. 41: 56. doi:10.15227/orgsyn.041.0056.
- ^ Nencki, M.; Sieber, N. (1881). "Über die Verbindungen der ein- und zweibasischen Fettsäuren mit Phenolen". Journal für Praktische Chemie. 23 (1): 147–156. doi:10.1002/prac.18810230111.
- ^ A. Seyewitz, Bull. Soc. Chins., 1890, 3, p. 811
- ^ Traub, M. C.; Hock, C. (1884). "Ueber ein Lakmoid". Berichte der Deutschen Chemischen Gesellschaft. 17 (2): 2615–2617. doi:10.1002/cber.188401702192.
- ^ J. T. Hewitt and F. G. Pope, Jour. C/tern. Soc., 1897, 75, p. 1084
- ^ Michael, Arthur (1888). "Ueber das Verhalten von Natriummalonäther gegen Resorcinol". Journal für Praktische Chemie. 37 (1): 469–471. doi:10.1002/prac.18880370144.
- ^ a b Cheng, H. A.; Drinnan, C. T.; Pleshko, N.; Fisher, O. Z. (21 October 2015). "Pseudotannins Self-assembled into Antioxidant Complexes". Soft Matter. 11 (39): 7783–7791. Bibcode:2015SMat...11.7783C. doi:10.1039/c5sm01224d. ISSN 1744-683X. PMC 4589535. PMID 26313262.
- ^ Suzuki, Y (1999). "Structures of 5-alkylresorcinol-related analogues in rye". Phytochemistry. 52 (2): 281–289. doi:10.1016/S0031-9422(99)00196-X.
- ^ Boer, J; Jemec, GB (2010). "Resorcinol peels as a possible self-treatment of painful nodules in hidradenitis suppurativa". Clinical and Experimental Dermatology. 35 (1): 36–40. doi:10.1111/j.1365-2230.2009.03377.x. PMID 19549239. S2CID 1794323.
- ^ Euresol, PubChem
- ^ Wipperman, J; Bragg, DA; Litzner, B (1 November 2019). "Hidradenitis Suppurativa: Rapid Evidence Review". American Family Physician. 100 (9): 562–569. PMID 31674740.
- ^ "Military Explosives," Department of the Army Technical Manual, TM-9-1300-214 (Washington, D.C.: Department of the Army, September 1984), p. 7-12.
- ^ Weselsky, P (1871). "Neue Derivate des Resorcins" [New derivatives of resorcinol]. Berichte der Deutschen Chemischen Gesellschaft. 4: 32–33. doi:10.1002/cber.18710040114.
- ^ Piccard, J.; Humbert, A. (1876). "Ueber eine Resorcindisulfosäure" [On a resorcinol disulfonic acid]. Berichte der Deutschen Chemischen Gesellschaft. 9 (2): 1479–1483. doi:10.1002/cber.187600902133.
- ^ a b Sidera, K.; Patsavoudi, E. (January 2014). "HSP90 inhibitors: current development and potential in cancer therapy". Recent Patents on Anti-Cancer Drug Discovery. 9 (1): 1–20. doi:10.2174/15748928113089990031. PMID 23312026.
- ^ Biamonte, M. A.; Van de Water, R.; Arndt, J. W.; Scannevin, R. H.; Perret, D.; Lee, W.-C. (January 2010). "Heat shock protein 90: Inhibitors in clinical trials". Journal of Medicinal Chemistry. 53 (1): 3–17. doi:10.1021/jm9004708. PMID 20055425.
- ^ Raj B. Durairaj. Resorcinol: Chemistry, Technology and Applications. Springer Science & Business Media, 2005 ISBN 9783540280903
- ^ McConnell, Virginia F. (1953). "Hlasiwetz and Barth — Pioneers in the structural aspects of plant products". Journal of Chemical Education. 30 (8): 380. Bibcode:1953JChEd..30..380M. doi:10.1021/ed030p380.
- ^ Panico, R.; & Powell, W. H. (Eds.) (1994). A Guide to IUPAC Nomenclature of Organic Compounds 1992. Oxford: Blackwell Science. ISBN 978-0-632-03488-8.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ H. Hlasiwetz and L. Barth (1864) "Ueber einen neuen, dem Orcin homologen Körper" (On a new substance homologous to orcin), Annalen der Chemie, 130 (3) : 354-359. Resorcinol is named on p. 358: "Wir nennen den neuen Körper, da wir gefunden haben, dass er auch aus dem Ammoniakgummiharz erhalten werden kann, Resorcin, um an seine Entstehung aus Harzen und seine Beziehung zum Orcin zu erinnern." (We name the new substance, since we have found that it can be obtained from ammoniated resin gum, resorcin, in order to recall its creation from resin and its relation to orcin.)
- ^ "Resorcinol to be added to REACH candidate list after long battle". Chemical Watch. 10 February 2022. Retrieved 16 August 2022.
External links
edit- International Chemical Safety Card 1033
- NIOSH Pocket Guide to Chemical Hazards
- IARC Monograph: "Resorcinol"
- IUPAC Nomenclature of Organic Chemistry (online version of the "Blue Book")
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Resorcin". Encyclopædia Britannica. Vol. 23 (11th ed.). Cambridge University Press. pp. 183–184.