Thiosulfate (IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula S2O2−3. Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, such as sodium thiosulfate Na2S2O3 and ammonium thiosulfate (NH4)2S2O3. Thiosulfate salts occur naturally. Thiosulfate rapidly dechlorinates water, and is used to halt bleaching in the paper-making industry. Thiosulfate salts are mainly used for dyeing in textiles, and bleaching of natural substances.[2]
| |

| |
| Names | |
|---|---|
IUPAC names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| S2O2−3 | |
| Molar mass | 112.12 g·mol−1 |
| Conjugate acid | Thiosulfuric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Structure and bonding
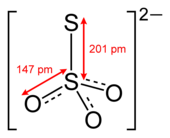
editThiosulfate is tetrahedral at the central S atom. Thiosulfate ion has C3v symmetry. The external sulfur atom has a valence of 2 while the central sulfur atom has a valence of 6. The oxygen atoms have a valence of 2. The S-S distance is appropriate for a single bond. The S-O distances are slightly shorter than the S-O distances in sulfate.
A longtime, the oxidation states of the two sulfur atoms in thiosulfate were considered to be +6 as in sulfate and −2 as in sulfide. This view precluded the disproportionation reaction of thiosulfate into sulfate and sulfide as a redox mechanism for providing energy to bacteria under anaerobic conditions in sediments. However, XANES spectroscopy measurements have revealed that the charge densities of both sulfur atoms point out to +5 and −1 oxidation states. This observation is consistent with the disproportionation of thiosulfate into sulfate and sulfide as a redox mechanism freeing up energy from microbial fermentation.[3]
Formation
editThiosulfate ion is produced by the reaction of sulfite ion with elemental sulfur, and by incomplete oxidation of sulfides (e.g. pyrite oxidation). Sodium thiosulfate can be formed by disproportionation of sulfur dissolving in sodium hydroxide (similar to phosphorus).
Reactions
editThiosulfate ions reacts with acids to give sulfur dioxide and various sulfur rings:[4]
- 8 S2O2−3 + 16 H+ → 8 SO2 + S8 + 8 H2O
This reaction may be used to generate sulfur colloids and demonstrate the Rayleigh scattering of light in physics. If white light is shone from below, blue light is seen from sideways and orange light from above, due to the same mechanisms that color the sky at midday and dusk.[citation needed]
Thiosulfate ions react with iodine to give tetrathionate ions:
- 2 S2O2−3 + I2 → S4O2−6 + 2 I−
This reaction is key for iodometry. With bromine (X = Br) and chlorine (X = Cl), thiosulfate ions are oxidized to sulfate ions:
- S2O2−3 + 4 X2 + 5 H2O → 2 SO2−4 + 8 X− + 10 H+
Reactions with metals and metal ions
editThiosulfate ion extensively forms diverse complexes with transition metals. This reactivity is related to its role in of silver-based photography.
Also reflecting its affinity for metals, thiosulfate ion rapidly corrodes metals in acidic conditions. Steel and stainless steel are particularly sensitive to pitting corrosion induced by thiosulfate ions. Molybdenum improves the resistance of stainless steel toward pitting (AISI 316L hMo). In alkaline aqueous conditions and medium temperature (60 °C), carbon steel and stainless steel (AISI 304L, 316L) are not attacked, even at high concentration of base (30%w KOH), thiosulfate ion (10%w) and in presence of fluoride ion (5%w KF).[citation needed]
In the era of silver-based photography, thiosulfate salts were consumed on a large scale as a "fixer" reagent. This application exploits thiosulfate ion's ability to dissolve silver halides. Sodium thiosulfate, commonly called hypo (from "hyposulfite"), was widely used in photography to fix black and white negatives and prints after the developing stage; modern "rapid" fixers use ammonium thiosulfate as a fixing salt because it acts three to four times faster.[5]
Thiosulfate salts have been used to extract or leach gold and silver from their ores as a less toxic alternative to cyanide ion.[2]
Biochemistry
editThe enzyme rhodanase (thiosulfate sulfurtransferase) catalyzes the detoxification of cyanide ion by thiosulfate ion by transforming them into thiocyanate ion and sulfite ion:
- CN− + S2O2−3 → SCN− + SO2−3
Sodium thiosulfate has been considered as an empirical treatment for cyanide poisoning, along with hydroxocobalamin. It is most effective in a pre-hospital setting, since immediate administration by emergency personnel is necessary to reverse rapid intracellular hypoxia caused by the inhibition of cellular respiration, at complex IV.[6][7][8][9]
It activates thiosulfate sulfurtransferase (TST) in mitochondria. TST is associated with protection against obesity and type II (insulin resistant) diabetes.[10][11]
Thiosulfate can also work as electron donor for growth of bacteria oxidizing sulfur, such as Chlorobium limicola forma thiosulfatophilum. These bacteria use electrons from thiosulfate (and other sources) and carbon from carbon dioxide to synthesize carbon compounds through reverse Krebs cycle.[12]
Some bacteria can metabolise thiosulfates.[13]
Minerals
editThiosulfate ion is a component of the very rare mineral sidpietersite Pb4(S2O3)O2(OH)2.[14] The presence of this anion in the mineral bazhenovite was disputed.[15]
Nomenclature
editThiosulfate is an acceptable common name and used almost always.
The functional replacement IUPAC name is sulfurothioate; the systematic additive IUPAC name is trioxidosulfidosulfate(2−) or trioxido-1κ3O-disulfate(S—S)(2−).[1]
Thiosulfate also refers to the esters of thiosulfuric acid, e.g. O,S-dimethyl thiosulfate CH3−O−S(=O)2−S−CH3. Such species are rare.
References
edit- ^ a b International Union of Pure and Applied Chemistry (2005). Nomenclature of Inorganic Chemistry (IUPAC Recommendations 2005). Cambridge (UK): RSC–IUPAC. ISBN 0-85404-438-8. pp. 139,329. Electronic version.
- ^ a b Barberá, J.J.; Metzger, A.; Wolf, M. (2000-06-15). "Sulfites, Thiosulfates, and Dithionites". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA. doi:10.1002/14356007.a25_477. ISBN 978-3-527-30673-2.
- ^ Vairavamurthy, A.; Manowitz, B.; Luther, G.W.; Jeon, Y. (1993). "Oxidation state of sulfur in thiosulfate and implications for anaerobic energy metabolism". Geochimica et Cosmochimica Acta. 57 (7). Elsevier BV: 1619–1623. doi:10.1016/0016-7037(93)90020-w. ISSN 0016-7037.
- ^ Steudel, Ralf (1982). "Homocyclic Sulfur Molecules". Inorganic Ring Systems. Topics in Current Chemistry. Vol. 102. pp. 149–176. doi:10.1007/3-540-11345-2_10. ISBN 978-3-540-11345-4.
- ^ Sowerby, A. L. M., ed. (1961). Dictionary of Photography: A Reference Book for Amateur and Professional Photographers (19th ed.). London: Illife Books Ltd.[page needed]
- ^ Hall, Alan H.; Dart, Richard; Bogdan, Gregory (2007). "Sodium Thiosulfate or Hydroxocobalamin for the Empiric Treatment of Cyanide Poisoning?". Annals of Emergency Medicine. 49 (6): 806–13. doi:10.1016/j.annemergmed.2006.09.021. PMID 17098327.
- ^ Hamel, J. (2011). "A Review of Acute Cyanide Poisoning with a Treatment Update" (PDF). Critical Care Nurse. 31 (1): 72–81, quiz 82. doi:10.4037/ccn2011799. PMID 21285466. Archived from the original (PDF) on 2013-06-12. Retrieved 2014-08-18.
- ^ Shepherd, G.; Vélez, L. I (2008). "Role of Hydroxocobalamin in Acute Cyanide Poisoning". Annals of Pharmacotherapy. 42 (5): 661–9. doi:10.1345/aph.1K559. PMID 18397973. S2CID 24097516.
- ^ Miles, Bryant (February 24, 2003). "Inhibitors & Uncouplers" (PDF). Texas A&M University. Archived from the original (PDF) on 4 March 2016. Retrieved 25 November 2015.
- ^ Stylianou, I. M.; et al. (2005). "Microarray gene expression analysis of the Fob3b obesity QTL identifies positional candidate gene Sqle and perturbed cholesterol and glycolysis pathways". Physiological Genomics. 20 (3): 224–232. CiteSeerX 10.1.1.520.5898. doi:10.1152/physiolgenomics.00183.2004. PMID 15598878.
- ^ Morton, N. M.; Beltram, J.; Carter, R. N.; et al. (2016). "Genetic identification of thiosulfate sulfurtransferase as an adipocyte-expressed antidiabetic target in mice selected for leanness". Nature Medicine. 22 (7): 771–779. doi:10.1038/nm.4115. PMC 5524189. PMID 27270587.
- ^ Buchanan, Bob B.; Arnon, Daniel I. (1990-04-01). "A reverse KREBS cycle in photosynthesis: consensus at last". Photosynthesis Research. 24 (1): 47–53. Bibcode:1990PhoRe..24...47B. doi:10.1007/BF00032643. ISSN 1573-5079. PMID 24419764. S2CID 2753977.
- ^ C.Michael Hogan. 2011. Sulfur. Encyclopedia of Earth, eds. A.Jorgensen and C.J.Cleveland, National Council for Science and the environment, Washington DC
- ^ handbookofmineralogy.org, Mineral Handbook, citing Roberts, A.C.; Cooper, M.A.; Hawthorne, F.C.; Stanley, C.J.; Key, C.L.; Jambor, J.L. (1999). "Sidpietersite, Pb4(S6+O3S2-)O2(OH)2, a new thiosulfate-bearing mineral species from Tsumeh, Namibia". The Canadian Mineralogist. 37: 1269-1273. and Cooper, M.A.; Hawthorne, F.C. (1999). "The structure and topology of sidpietersite,Pb4(S6+O3S2-)O2(OH)2". The Canadian Mineralogist. 37: 1275-1283.
- ^ Bindi, Luca; Bonazzi, Paola; Dei, Luigi; Zoppi, Angela (2005). "Does the bazhenovite structure really contain a thiosulfate group? A structural and spectroscopic study of a sample from the type locality". American Mineralogist. 90 (10): 1556–1562. Bibcode:2005AmMin..90.1556B. doi:10.2138/am.2005.1781. S2CID 59941277.