This article needs additional citations for verification. (July 2022) |
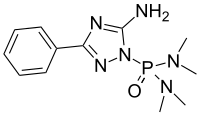
Triamiphos (chemical formula: C12H19N6OP) is an organophosphate used as a pesticide and fungicide. It is used to control powdery mildews on apples and ornamentals. It was discontinued by the US manufacturer in 1998.[1]
| |
| Names | |
|---|---|
| Preferred IUPAC name
P-(5-Amino-3-phenyl-1H-1,2,4-triazol-1-yl)-N,N,N′,N′-tetramethylphosphonic diamide | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.149.164 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H19N6OP | |
| Molar mass | 294.299 g·mol−1 |
| Appearance | white powder |
| 0.25 g/L | |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H300, H310 | |
| P262, P264, P270, P280, P301+P310, P302+P350, P310, P321, P322, P330, P361, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
History
editThe phosphoramide Triamiphos is thought to be the first commercially available systemic fungicide.[2] Despite its prominent use in the years following its discovery, no long-term toxicity studies were undertaken until 1974.[3] Further, it has since been replaced by other pesticides. The WHO recommended classification of pesticides by hazard considers triamiphos to be discontinued as use for pesticide.[4]
Structure and Reactivity
editIt is classified as an organophosphorus compound O=P(R)3 and more specifically as a phosphoramide O=P(NR2)3. The bis(dimethylamido)phosphoryl group (Me2N)2-P(O)- is present in triamiphos and also a number of other fungicides.[2]
It contains two chemical groups used in pesticide synthesis (triazole, phosphoryl). The most relevant distinct subparts of the molecule are the oxon centre (O=P) and the leaving group (the triazole aromatic moiety). Triamiphos technically is not an organophosphate O=P(OR)3, a subclass of organophosphorus O=PR3 compounds. However, the distinction is not always consistent throughout literature where organophosphorus compounds without the alkoxy sidechains or even with a O=S group instead of a O=P group are still classified as organophosphate pesticides (OPs).[5][6]
Schradan, another organophosphorus pesticide, can be seen as analogous to triamiphos, differing only in the leaving group. As both have comparable toxic properties, it can be concluded that the phenylaminotriazole moiety of triamiphos does not appear to be vital for its anticholinesterase property.[3]
Synthesis
editTriamiphos was first synthesised by Van den Bos et al. (1960) by adding the salt of 3-amino-5-phenyl-1,2,4-triazole to a solution of phosphoryl chloride. Subsequently, gaseous dimethylamine is introduced into the reaction mixture to yield triamiphos.[7]
Biotransformation
editThere were no studies on the exact determination of the biotransformation route and the active metabolite’s structure of Triamiphos.
Mechanism of Action and Toxicity in Animal Studies
editThe toxic effect of Triamiphos ties back to the acetylcholinesterase inhibition ability of its active metabolite.[8][9] This inhibitory effect is observed for absorption routes through the skin, respiratory or digestive tract.[10]
The National Institute of Public Health in The Netherlands reported a dose-dependent effect of Triamiphos from a short-term study in rats. They found inhibition of acetylcholinesterase activity at a concentration of 1 ppm during the feeding period. After a recovery period the enzyme activity returned back to normal.[11] A long-term feeding and a three-generation reproduction study performed by Verschuuren et al. (1974), however, found inhibitory effects at an even lower concentration of 0.5 ppm. At this concentration, cholinesterase activity was inhibited in the P-, but not in the F1, F2 or F3 generations. Inhibition in all generations was observed at a concentration of 2.5 ppm, in which the subsequent generations were already exposed to the toxicant from the moment of conception.[3]
A no-effect level of 0.1 ppm was reported by both studies.[3][11]
Furthermore, a greater inhibitory effect on erythrocyte cholinesterase compared to plasma or brain cholinesterase activity was reported.[3] Therefore, the active metabolite does not appear to readily enter the brain and primarily muscarinic and nicotinic effects are observed.[8] The LD50 (i.p. route) was determined to be between 15 and 18 mg/kg in rats and 10–30 mg/kg in mice.[3][8][12] Animals receiving the lethal dose were reported to be maintained upon administration of atropine as antidote.[8] An important factor responsible for the acute toxicity of Triamiphos is the rate of cholinesterase inhibition: if the activity is reduced by 70% within a few minutes, death primarily due to paralysis of the respiratory muscles in rats was reported. The inhibited enzyme is not reactivated and the above-mentioned recovery of the animals was only possible due to its resynthesis.[8] A 1976-study suggested an increased cholesterol content in rat aorta and changes in lipid metabolism as further effects of Triamiphos, which could however not be confirmed by another, more elaborate study.[13][14] An overview of the effects of Triamiphos at different concentrations can be found in the table below.
| Dose | Effect |
|---|---|
| 0.1 ppm | NOAEL (no-effect level)[3][11] |
| 0.5 ppm | Inhibitory effects in P-generation[3] |
| 2.5 ppm | Inhibitory effects in P- and subsequent generations[3] |
| 15–18 mg/kg | Oral LD50 in rats[3][8] |
| 10–30 mg/kg | Oral LD50 in mice[12] |
Indications
editTriamiphos is suspected to exert the same toxic side effects to humans as other organophosphorus pesticides, though no human data on specifically Triamiphos exposure seems to be available.[15]
References
edit- ^ "TRIAMIPHOS | CAMEO Chemicals | NOAA". cameochemicals.noaa.gov. Retrieved 2022-07-04.
- ^ a b Mavrommatis, Christakis Nikou (November 1983). The synthesis, spectroscopy and fungicidal activity of phosphoric acid amides (doctoral thesis). Polytechnic of North London.
- ^ a b c d e f g h i j Verschuuren, H.G.; Kroes, R. (December 1974). "Triamiphos: Long-term toxicity and three-generation reproduction studies in rats". Toxicology. 2 (4): 327–338. doi:10.1016/0300-483X(74)90025-0. PMID 4855258.
- ^ The WHO recommended classification of pesticides by hazard and guidelines to classification 2019. Geneva: WHO. 2020. ISBN 978-92-4-000566-2.
- ^ Ganie, Shahid Yousuf; Javaid, Darakhshan; Hajam, Younis Ahmad; Reshi, Mohd. Salim (2022-04-30). "Mechanisms and treatment strategies of organophosphate pesticide induced neurotoxicity in humans: A critical appraisal". Toxicology. 472: 153181. doi:10.1016/j.tox.2022.153181. ISSN 0300-483X. PMID 35439576. S2CID 248220887.
- ^ Buchet, J. P.; Roels, H.; Lauwerys, R. (1974-01-16). "Further characterization of mono and diglyceride lipases in rat tissues". Life Sciences. 14 (2): 371–385. doi:10.1016/0024-3205(74)90068-X. ISSN 0024-3205. PMID 4360447.
- ^ van den Bos, B. G.; Koopmans, M. J.; Huisman, H. O. (2010-09-02). "Investigations on pesticidal phosphorus compounds I. Fungicides, insecticides and acaricides derived from 3-amino-1,2,4-triazole". Recueil des Travaux Chimiques des Pays-Bas. 79 (8): 807–822. doi:10.1002/recl.19600790806.
- ^ a b c d e f Lauwerys, R.; Buchet, J. -P. (1971-11-01). "Studies on the mechanism of toxicity of the organophosphorus pesticide triamiphos". European Journal of Pharmacology. 16 (3): 361–366. doi:10.1016/0014-2999(71)90039-2. ISSN 0014-2999. PMID 5132563.
- ^ Kwong, Tai C. (February 2002). "Organophosphate Pesticides: Biochemistry and Clinical Toxicology". Therapeutic Drug Monitoring. 24 (1): 144–149. doi:10.1097/00007691-200202000-00022. ISSN 0163-4356. PMID 11805735. S2CID 22423462.
- ^ Spencer, E.Y. (1982). "Guide to the Chemicals Used in Crop Protection". Agriculture Canada. 165.
- ^ a b c Unpublished Reports. Bilthoven: RIVM. 1962.
- ^ a b Tolkmith, Henry (April 1966). "Acute Mammalian Toxicity and Structure of Heterocyclic Organophosphorus Compounds". Annals of the New York Academy of Sciences. 136 (3 Acute Mammali): 61–94. Bibcode:1966NYASA.136...61T. doi:10.1111/j.1749-6632.1966.tb31409.x. ISSN 0077-8923. S2CID 83584149.
- ^ Lauwerys, R. R.; Buchet, J. P.; Roels, H. (1976). "The relationship between cadmium exposure or body burden and the concentration of cadmium in blood and urine in man". International Archives of Occupational and Environmental Health. 36 (4): 275–285. Bibcode:1976IAOEH..36..275L. doi:10.1007/BF00409357. ISSN 0340-0131. PMID 1254345. S2CID 8988637.
- ^ Buchet, J. P.; Lauwerys, R.; Roels, H. (February 1977). "Long term exposure to organophosphorus pesticides and lipid metabolism in the rat". Bulletin of Environmental Contamination and Toxicology. 17 (2): 175–183. Bibcode:1977BuECT..17..175B. doi:10.1007/BF01685547. ISSN 0007-4861. PMID 66077. S2CID 13100142.
- ^ Barlow, Susan M.; Sullivan, Frank M.; Miller, Richard K. (2015-01-01), Schaefer, Christof; Peters, Paul; Miller, Richard K. (eds.), "2.23 - Occupational, industrial and environmental agents", Drugs During Pregnancy and Lactation (Third Edition), San Diego: Academic Press, pp. 599–638, doi:10.1016/b978-0-12-408078-2.00024-x, ISBN 978-0-12-408078-2, retrieved 2023-03-18