The immunoglobulin superfamily (IgSF) is a large protein superfamily of cell surface and soluble proteins that are involved in the recognition, binding, or adhesion processes of cells. Molecules are categorized as members of this superfamily based on shared structural features with immunoglobulins (also known as antibodies); they all possess a domain known as an immunoglobulin domain or fold. Members of the IgSF include cell surface antigen receptors, co-receptors and co-stimulatory molecules of the immune system, molecules involved in antigen presentation to lymphocytes, cell adhesion molecules, certain cytokine receptors and intracellular muscle proteins. They are commonly associated with roles in the immune system. Otherwise, the sperm-specific protein IZUMO1, a member of the immunoglobulin superfamily, has also been identified as the only sperm membrane protein essential for sperm-egg fusion.
| Immunoglobulin superfamily | |||||||||
|---|---|---|---|---|---|---|---|---|---|
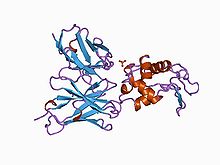 Antibody in complex with hen egg white lysozyme.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | IgSF | ||||||||
| Pfam | PF00047 | ||||||||
| Pfam clan | CL0159 | ||||||||
| ECOD | 11.1.1 | ||||||||
| InterPro | IPR013151 | ||||||||
| PROSITE | PS50835 | ||||||||
| SCOP2 | 1tlk / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 193 | ||||||||
| OPM protein | 3bib | ||||||||
| CDD | cd00096 | ||||||||
| Membranome | 2 | ||||||||
| |||||||||
| Immunoglobulin-like (ligands) | |
|---|---|
| Identifiers | |
| Symbol | Ig protein ligands |
| Membranome | 64 |
| Immunoglobulin-like adhesion molecules | |
|---|---|
| Identifiers | |
| Symbol | IgSF CAM |
| Membranome | 110 |
Immunoglobulin domains
editProteins of the IgSF possess a structural domain known as an immunoglobulin (Ig) domain. Ig domains are named after the immunoglobulin molecules. They contain about 70-110 amino acids and are categorized according to their size and function.[2] Ig-domains possess a characteristic Ig-fold, which has a sandwich-like structure formed by two sheets of antiparallel beta strands. Interactions between hydrophobic amino acids on the inner side of the sandwich and highly conserved disulfide bonds formed between cysteine residues in the B and F strands, stabilize the Ig-fold.[citation needed]
Classification
editThe Ig like domains can be classified as IgV, IgC1, IgC2, or IgI.[3]
Most Ig domains are either variable (IgV) or constant (IgC).
- IgV: IgV domains with 9 beta strands are generally longer than IgC domains with 7 beta strands.
- IgC1 and IgC2: Ig domains of some members of the IgSF resemble IgV domains in the amino acid sequence, yet are similar in size to IgC domains. These are called IgC2 domains, while standard IgC domains are called IgC1 domains.
- IgI: Other Ig domains exist that are called intermediate (I) domains.[4]
Members
editThe Ig domain was reported to be the most populous family of proteins in the human genome with 765 members identified.[5] Members of the family can be found even in the bodies of animals with a simple physiological structure such as poriferan sponges. They have also been found in bacteria, where their presence is likely to be due to divergence from a shared ancestor of eukaryotic immunoglobulin superfamily domains.[6]
| Molecule function/category | Examples | Description |
|---|---|---|
| Antigen receptors |
|
Antigen receptors found on the surface of T and B lymphocytes in all jawed vertebrates belong to the IgSF. Immunoglobulin molecules (the antigen receptors of B cells) are the founding members of the IgSF. In humans, there are five distinct types of immunoglobulin molecule all containing a heavy chain with four Ig domains and a light chain with two Ig domains. The antigen receptor of T cells is the T-cell receptor (TCR), which is composed of two chains, either the TCR-alpha and -beta chains, or the TCR-delta and gamma chains. All TCR chains contain two Ig domains in the extracellular portion; one IgV domain at the N-terminus and one IgC1 domain adjacent to the cell membrane. |
| Antigen presenting molecules | The ligands for TCRs are major histocompatibility complex (MHC) proteins. These come in two forms; MHC class I forms a dimer with a molecule called beta-2 microglobulin (β2M) and interacts with the TCR on cytotoxic T cells and MHC class II has two chains (alpha and beta) that interact with the TCR on helper T cells. MHC class I, MHC class II and β2M molecules all possess Ig domains and are therefore also members of the IgSF. | |
| Co-receptors | Co-receptors and accessory molecules: Other molecules on the surfaces of T cells also interact with MHC molecules during TCR engagement. These are known as co-receptors. In lymphocyte populations, the co-receptor CD4 is found on helper T cells and the co-receptor CD8 is found on cytotoxic T cells. CD4 has four Ig domains in its extracellular portion and functions as a monomer. CD8, in contrast, functions as a dimer with either two identical alpha chains or, more typically, with an alpha and beta chain. CD8-alpha and CD8-beta each has one extracellular IgV domain in its extracellular portion. A co-receptor complex is also used by the BCR, including CD19, an IgSF molecule with two IgC2-domains. | |
| Antigen receptor accessory molecules | A further molecule is found on the surface of T cells that is also involved in signaling from the TCR. CD3 is a molecule that helps to transmit a signal from the TCR following its interaction with MHC molecules. Three different chains make up CD3 in humans, the gamma chain, delta chain and epsilon chain, all of which are IgSF molecules with a single Ig domain.
Similar to the situation with T cells, B cells also have cell surface co-receptors and accessory molecules that assist with cell activation by the B Cell Receptor (BCR)/immunoglobulin. Two chains are used or signaling, CD79a and CD79b that both possess a single Ig domain. | |
| Co-stimulatory or inhibitory molecules | Co-stimulatory or inhibitory molecules: Co-stimulatory and inhibitory signaling receptors and ligands control the activation, expansion and effector functions of cells. One major group of IgSF co-stimulatory receptors are molecules of the CD28 family; CD28, CTLA-4, program death-1 (PD-1), the B- and T-lymphocyte attenuator (BTLA, CD272), and the inducible T-cell co-stimulator (ICOS, CD278);[7] and their IgSF ligands belong to the B7 family; CD80 (B7-1), CD86 (B7-2), ICOS ligand, PD-L1 (B7-H1), PD-L2 (B7-DC), B7-H3, and B7-H4 (B7x/B7-S1).[8] | |
| Receptors on Natural killer cells | ||
| Receptors on Leukocytes | ||
| IgSF CAMs | ||
| Cytokine receptors | ||
| Growth factor receptors |
| |
| Receptor tyrosine kinases/phosphatases |
| |
| Ig binding receptors |
| |
| Cytoskeleton | ||
| Others |
|
References
edit- ^ Dall'Acqua W, Goldman ER, Lin W, Teng C, Tsuchiya D, Li H, Ysern X, Braden BC, Li Y, Smith-Gill SJ, Mariuzza RA (June 1998). "A mutational analysis of binding interactions in an antigen-antibody protein-protein complex". Biochemistry. 37 (22): 7981–91. doi:10.1021/bi980148j. PMID 9609690.
- ^ Barclay AN (August 2003). "Membrane proteins with immunoglobulin-like domains--a master superfamily of interaction molecules". Seminars in Immunology. 15 (4): 215–23. doi:10.1016/S1044-5323(03)00047-2. PMID 14690046.
- ^ B. D. Gomperts; Ijsbrand M. Kramer; Peter E. R. Tatham (1 July 2009). Signal transduction. Academic Press. pp. 378–. ISBN 978-0-12-369441-6. Retrieved 28 November 2010.
- ^ Harpaz Y, Chothia C (May 1994). "Many of the immunoglobulin superfamily domains in cell adhesion molecules and surface receptors belong to a new structural set which is close to that containing variable domains". Journal of Molecular Biology. 238 (4): 528–39. doi:10.1006/jmbi.1994.1312. PMID 8176743.
- ^ Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. (February 2001). "Initial sequencing and analysis of the human genome" (PDF). Nature. 409 (6822): 860–921. doi:10.1038/35057062. PMID 11237011.
- ^ Bateman A, Eddy SR, Chothia C (September 1996). "Members of the immunoglobulin superfamily in bacteria". Protein Science. 5 (9): 1939–41. doi:10.1002/pro.5560050923. PMC 2143528. PMID 8880921.
- ^ Peggs KS, Allison JP (September 2005). "Co-stimulatory pathways in lymphocyte regulation: the immunoglobulin superfamily". British Journal of Haematology. 130 (6): 809–24. doi:10.1111/j.1365-2141.2005.05627.x. PMID 16156851.
- ^ Greenwald RJ, Freeman GJ, Sharpe AH (2005). "The B7 family revisited". Annual Review of Immunology. 23: 515–48. doi:10.1146/annurev.immunol.23.021704.115611. PMID 15771580.
- ^ Boles KS, Stepp SE, Bennett M, Kumar V, Mathew PA (June 2001). "2B4 (CD244) and CS1: novel members of the CD2 subset of the immunoglobulin superfamily molecules expressed on natural killer cells and other leukocytes". Immunological Reviews. 181: 234–49. doi:10.1034/j.1600-065X.2001.1810120.x. PMID 11513145. S2CID 21801197.
- ^ Fraser CC, Howie D, Morra M, Qiu Y, Murphy C, Shen Q, Gutierrez-Ramos JC, Coyle A, Kingsbury GA, Terhorst C (February 2002). "Identification and characterization of SF2000 and SF2001, two new members of the immune receptor SLAM/CD2 family". Immunogenetics. 53 (10–11): 843–50. doi:10.1007/s00251-001-0415-7. PMID 11862385. S2CID 10257502.
- ^ Tangye SG, Nichols KE, Hare NJ, van de Weerdt BC (September 2003). "Functional requirements for interactions between CD84 and Src homology 2 domain-containing proteins and their contribution to human T cell activation". Journal of Immunology. 171 (5): 2485–95. doi:10.4049/jimmunol.171.5.2485. PMID 12928397.
External links
edit- Transmembrane human proteins from immunoglobulin superfamily classified as receptors, ligands and adhesion proteins
- Immunoglobulin domain in SUPERFAMILY