Lubiprostone, sold under the brand name Amitiza among others, is a medication used in the management of chronic idiopathic constipation, predominantly irritable bowel syndrome-associated constipation in women and opioid-induced constipation. The drug is owned by Mallinckrodt and is marketed by Takeda Pharmaceutical Company.
 | |
| Clinical data | |
|---|---|
| Trade names | Amitiza |
| Other names | RU-0211 SPI-0211 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607034 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Negligible |
| Protein binding | 94% |
| Metabolism | Extensive, CYP not involved |
| Elimination half-life | Unknown (lubiprostone) 0.9–1.4 hours (main metabolite) |
| Excretion | Kidney (60%) and fecal (30%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.107.168 |
| Chemical and physical data | |
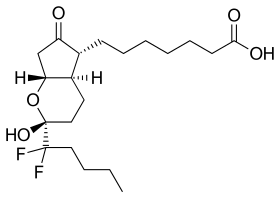
| Formula | C20H32F2O5 |
| Molar mass | 390.468 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
The drug was developed by Sucampo Pharmaceuticals and approved by the Food and Drug Administration (FDA) in 2006.[2][3][4] It was recommended for use in the UK by the National Institute for Health and Care Excellence (NICE) in July 2014.[5] Health Canada approved the drug in 2015.[6] Lubiprostone received approval from the Food and Drug Administration in 2008, to treat irritable bowel syndrome with constipation (IBS-C),[7] and in 2013, for the treatment of opioid-induced constipation in adults with chronic noncancer pain.[4] It is available as a generic medication.[8]
Medical uses
editLubiprostone is a laxative used for the treatment of constipation, specifically:[9]
- Chronic idiopathic constipation (difficult or infrequent passage of stools that lasts for 3 months or longer and is not caused by diet, disease, or drugs).[9][3][10][11][12][13][14]
- Constipation caused by certain opioid (narcotic) pain medications in people with chronic (ongoing), noncancer pain,[9] or in patients with long-lasting pain caused by a previous cancer or its treatment who do not need weekly increases in opioid dosage.[4][10][12][13]
- Irritable bowel syndrome with constipation (IBS-C; a condition that causes stomach pain or cramps, bloating, and infrequent or difficult passage of stools) in women who are at least 18 years of age.[9][3][7][10][11][12][13][14]
Lubiprostone has not been studied in children.[10][12] There is current research under way to determine the safety and efficacy in postoperative bowel dysfunction.
It comes in a liquid filled capsule and is available only with a doctor's prescription.[10] If one misses a dose it should be taken as soon as possible unless it is almost time for the next dose, in which case it should be skipped and the user should return to their regular dosing schedule.[10]
Adverse effects
editIn clinical trials, the most common adverse event was nausea (31%). Other adverse events (≥5% of patients) included diarrhea (13%), headache (13%), abdominal distension (5%), abdominal pain (5%), flatulence (6%), sinusitis (5%), vomiting (5%), and fecal incontinence (1%).
The FDA lists the following:[3]
For subjects with chronic idiopathic constipation taking Amitiza:
- Nausea ~ 29% (4% were severe, and 9% of patients discontinued treatment due to nausea. The rate of nausea was lower among male (8%) and elderly (19%) patients. No patients in the clinical studies were hospitalized due to nausea.)
- Diarrhea: ~12% (2% were severe, and 2% of patients discontinued treatment due to diarrhea)
- Several less common adverse reactions (<1%).
For opioid-induced constipation:
- Nausea: ~ 11%; 1% severe nausea and 2% discontinued treatment due to nausea.
- Diarrhea: ~ 8%; 2% severe diarrhea and 1% of patients discontinued treatment due to diarrhea.
- Less common adverse reactions (<1%): fecal incontinence, blood potassium decreased.
For subjects with irritable bowel syndrome with constipation:
- Nausea: ~ 8%; 1% severe nausea and 1% discontinued treatment due to nausea.
- Diarrhea: ~ 7%; <1% of patients had severe diarrhea and <1% of patients discontinued treatment due to diarrhea.
- Less common adverse reactions: <1%[3]
A 2018 pooled analysis from three phase III, randomized, double-blind, placebo-controlled studies on usage for Opioid-Induced Constipation, found that the numbers of patients reporting adverse effects were similar in both the lubiprostone and placebo treatment groups for all opioid classes (P ≥ 0.125); however, gastrointestinal adverse effects were reported more frequently by those receiving lubiprostone than 2 of the 3 opioid groups. The most commonly reported TEAEs in the lubiprostone treatment groups were nausea (13.4%–18.1%), diarrhea (1.2%–13.9%), and abdominal pain (4.7%–5.6%). In the population overall, the greatest likelihood of experiencing the first episode of any of these three TEAEs was greatest in the first week of treatment and decreased thereafter.[4]
According to Medscape, the most common (>10%) were: Nausea, Diarrhea (7-12%), Headache (2-11%). Less common side effects (1-10%) included: Abdominal pain (4-8%), Abdominal distension (3-6%), Flatulence (4-6%), Vomiting (3%), Loose stools (3%), Edema (1-3%), Abdominal discomfort (1-3%), Dizziness (3%), Chest discomfort/pain (2%), Dyspnea (2%), Dyspepsia (2%), Fatigue (2%), Dry mouth (1%).[13]
Contraindications
edit- Known or suspected mechanical GI obstruction.[3][14][12]
- Known hypersensitivity to lubiprostone or any ingredient in the formulation.[14]
The effects on pregnancy have not been studied in humans, but testing in guinea pigs resulted in fetal loss.[medical citation needed]
Lubiprostone is contraindicated in people exhibiting chronic diarrhea, bowel obstruction, or diarrhea-predominant irritable bowel syndrome.[medical citation needed]
Mechanism of action
editLubiprostone is a bicyclic fatty acid[15] derived from prostaglandin E1 that acts by specifically activating ClC-2 chloride channels on the apical aspect of gastrointestinal epithelial cells, producing a chloride-rich fluid secretion. These secretions soften the stool, increase motility, and promote spontaneous bowel movements.
Pharmacokinetics
editUnlike many laxative products, lubiprostone does not show signs of drug tolerance, chemical dependency, or altered serum electrolyte concentration.[16]
Minimal distribution of the drug occurs beyond the immediate gastrointestinal tissues.[medical citation needed] Lubiprostone is rapidly metabolized by reduction/oxidation, mediated by carbonyl reductase.[medical citation needed] There is no metabolic involvement of the hepatic cytochrome P450 system.[medical citation needed] The measurable metabolite, M3, exists in very low levels in plasma and makes up less than 10% of the total administered dose.[medical citation needed]
Data indicate that metabolism occurs locally in the stomach and jejunum.[medical citation needed]
Society and culture
editEconomics
editThe cost to the NHS was £29.68 per 24 mcg 28-cap pack as of April 2017.
Brand names
editLubiprostone is available in the United States, Japan, Switzerland, India, Bangladesh, the United Kingdom, and Canada.[citation needed]
In Bangladesh and India, lubiprostone is sold under the brand name Lubigut by Ziska Pharmaceuticals, Lubilax by Beacon Pharmaceuticals, and under the brand name Lubowel by Sun Pharmaceutical.[citation needed]
References
edit- ^ "Health Canada New Drug Authorizations: 2015 Highlights". Health Canada. 4 May 2016. Retrieved 7 April 2024.
- ^ "FDA Approves New Type of Drug To Treat Constipation in Adults". The Wall Street Journal. February 1, 2006.
- ^ a b c d e f g "Highlights of Prescribing Information" (PDF). FDA. 2020.
- ^ a b c d Webster LR, Brewer RP, Lichtlen P, Losch-Beridon T, Mareya S, Wang M (June 2018). "Efficacy of Lubiprostone for the Treatment of Opioid-Induced Constipation, Analyzed by Opioid Class". Pain Medicine. 19 (6): 1195–1205. doi:10.1093/pm/pnx212. PMID 29897589.
- ^ "Final appraisal determination: Lubiprostone for treating chronic idiopathic constipation". National Institute for Health and Care Excellence. June 2014.
- ^ "Health Canada New Drug Authorizations: 2015 Highlights". Health Canada. 2016-05-04.
- ^ a b "In the news: FDA approves one drug for irritable bowel syndrome but suspends another". Harvard Health. 2008-08-01.
- ^ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.
- ^ a b c d
- "Lubiprostone: MedlinePlus Drug Information". medlineplus.gov. 2017.
Lubiprostone is also used to treat irritable bowel syndrome with constipation... in women who are at least 18 years of age.
- "Lubiprostone Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing". WebMD.
- "Lubiprostone: MedlinePlus Drug Information". medlineplus.gov. 2017.
- ^ a b c d e f "Lubiprostone (Oral Route) Side Effects". Mayo Clinic. 2021.
- ^ a b Li F, Fu T, Tong WD, Liu BH, Li CX, Gao Y, et al. (April 2016). "Lubiprostone Is Effective in the Treatment of Chronic Idiopathic Constipation and Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Randomized Controlled Trials". Mayo Clinic Proceedings. 91 (4): 456–468. doi:10.1016/j.mayocp.2016.01.015. PMID 27046523.
Lubiprostone is a safe and efficacious drug for the treatment of chronic idiopathic constipation and irritable bowel syndrome with constipation, with limited adverse effects in 3 months of follow-up.
- ^ a b c d e f "Amitiza (Lubiprostone): Uses, Dosage, Side Effects, Interactions, Warning". RxList.
- ^ a b c d "Amitiza (lubiprostone) dosing, indications, interactions, adverse effects, and more". reference.medscape.com.
- ^ a b c d "Amitiza". The American Society of Health-System Pharmacists.
- ^ Lacy BE, Levy LC (April 2007). "Lubiprostone: a chloride channel activator". Journal of Clinical Gastroenterology. 41 (4): 345–351. doi:10.1097/01.mcg.0000225665.68920.df. PMID 17413599.
- ^ Lacy BE, Levy LC (June 2008). "Lubiprostone: a novel treatment for chronic constipation". Clinical Interventions in Aging. 3 (2): 357–364. doi:10.2147/cia.s2938. PMC 2546479. PMID 18686757.