| |
| |
| Names | |
|---|---|
| IUPAC name
iron(2+) lithium phosphate (1:1:1)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| FeLiO 4P | |
| Molar mass | 157.757 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium iron oxide is an inorganic compound with the formula NaFeO
2. It is a gray, red-grey, brown or black solid that is insoluble in water. The material has attracted attention as a component of sodium-ion batteries[1]. This battery chemistry is targeted for use in power tools, electric vehicles, solar energy installations[2] and more recently large grid-scale energy storage.[3]
Most lithium batteries (Li-ion) used in consumer electronics products use cathodes made of lithium compounds such as lithium cobalt oxide (LiCoO
2), lithium manganese oxide (LiMn
2O
4), and lithium nickel oxide (LiNiO
2). The anodes are generally made of graphite.
Lithium iron phosphate exists naturally in the form of the mineral triphylite, but this material has insufficient purity for use in batteries.
LiMPO
4
edit
With general chemical formula of LiMPO
4, compounds in the LiFePO
4 family adopt the olivine structure. M includes not only Fe but also Co, Mn and Ti.[4] As the first commercial LiMPO
4 was C/LiFePO
4, the whole group of LiMPO
4 is informally called “lithium iron phosphate” or “LiFePO
4”. However, more than one olivine-type phase may be used as a battery's cathode material. Olivine compounds such as A
yMPO
4, Li
1−xMFePO
4, and LiFePO
4−zM have the same crystal structures as LiMPO
4, and may replace it in a cathode. All may be referred to as “LFP”.
Manganese, phosphate, iron, and lithium also form an olivine structure. This structure is a useful contributor to the cathode of lithium rechargeable batteries.[5] This is due to the olivine structure created when lithium is combined with manganese, iron, and phosphate (as described above). The olivine structures of lithium rechargeable batteries are significant, for they are affordable, stable, and can be safely used to store energy.[6]
History and production
editArumugam Manthiram and John B. Goodenough first identified the polyanion class of cathode materials for lithium ion batteries.[7][8][9] LiFePO
4 was then identified as a cathode material belonging to the polyanion class for use in batteries in 1996 by Padhi et al.[10][11] Reversible extraction of lithium from LiFePO
4 and insertion of lithium into FePO
4 was demonstrated. Neutron diffraction confirmed that LFP was able to ensure the security of large input/output current of lithium batteries.[12]
The material can be produced by heating a variety of iron and lithium salts with phosphates or phosphoric acid. Many related routes have been described including those that use hydrothermal synthesis.[13]
Physical and chemical properties
editIn LiFePO
4, lithium has a +1 charge, iron +2 charge balancing the −3 charge for phosphate. Upon removal of Li, the material converts to the ferric form FePO
4.[14]
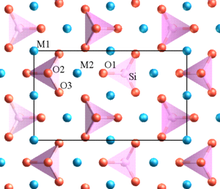
The iron atom and 6 oxygen atoms form an octahedral coordination sphere, described as FeO
6, with the Fe ion at the center. The phosphate groups, PO
4, are tetrahedral. The three-dimensional framework is formed by the FeO
6 octahedra sharing O corners. Lithium ions reside within the octahedral channels in a zigzag manner. In crystallography, this structure is thought to belong to the Pmnb space group of the orthorhombic crystal system. The lattice constants are: a = 6.008 Å, b = 10.334 Å, and c = 4.693 Å. The volume of the unit cell is 291.4 Å3.
In contrast to two traditional cathode materials, LiMnO
4 and LiCoO
2, lithium ions of LiMPO
4 migrate in the lattice's one-dimensional free volume. During charge/discharge, the lithium ions are extracted concomitant with oxidation of Fe:
Extraction of lithium from LiFePO
4 produces FePO
4 with a similar structure. FePO
4 adopts a Pmnb space group with a unit cell volume of 272.4 Å3, only slightly smaller than that of its lithiated precursor. Extraction of lithium ions reduces the lattice volume, as is the case with lithium oxides. LiMPO
4's corner-shared FeO
6 octahedra are separated by the oxygen atoms of the PO3−
4 tetrahedra and cannot form a continuous FeO
6 network, reducing conductivity.
A nearly close-packed hexagonal array of oxides centers provides relatively little free volume for Li+
ions to migrate within. For this reason, the ionic conductivity of Li+
is relatively low at ambient temperature. The details of the lithiation of FePO
4 and the delithiation of LiFePO
4 have been examined. Two phases of the lithiated material are implicated.[14][15]
Applications
editLFP cells have an operating voltage of 3.3 V, charge density of 170 mAh/g, high power density, long cycle life and stability at high temperatures.
LFP's major commercial advantages are that it poses few safety concerns such as overheating and explosion, as well as long cycle lifetimes, high power density and has a wider operating temperature range. Power plants and automobiles use LFP.[16][17]
BAE has announced that their HybriDrive Orion 7 hybrid bus uses about 180 kW LFP battery cells. AES has developed multi-trillion watt battery systems that are capable of subsidiary services of the power network, including spare capacity and frequency adjustment. In China, BAK and Tianjin Lishen are active in the area.
Comparison
editAlthough LFP has 25% less specific charge (Ah/g) than lithium batteries with oxide (e.g. nickel-cobalt-manganese, NCM) cathode materials due to its operational voltage (3.2 volts vs 3.7 for NCM-type cathode chemistries), it has 70% more than nickel-hydrogen batteries.
The major differences between LFP batteries and other lithium ion battery types is that LFP batteries contain no cobalt (removing ethical and economic questions about cobalt's availability) and have a flat discharge curve.
LFP batteries have drawbacks, originating from a high electronic resistivity of LFP, as well as the lower maximum charge/discharge voltage. The energy density is significantly lower than LiCoO
2 (although higher than the nickel–metal hydride battery).
Lithium cobalt oxide based battery chemistries are more prone to thermal runaway if overcharged and cobalt is both expensive and not widely geographically available. Other chemistries such as nickel-manganese-cobalt (NMC) have supplanted LiCo chemistry cells in most applications. The original ratio of Ni to Mn to Co was 3:3:3, whereas today, cells are being made with ratios of 8:1:1 or 6:2:2, whereby the Co content has been drastically reduced.
LiFePO4 batteries are comparable to sealed lead acid batteries and are often being touted as a drop-in replacement for lead acid applications. The most notable difference between lithium iron phosphate and lead acid is the fact that the lithium battery capacity shows only a small dependence on the discharge rate. With very high discharge rates, for instance 0.8C, the capacity of the lead acid battery is only 60% of the rated capacity. Therefore, in cyclic applications where the discharge rate is often greater than 0.1C, a lower rated lithium battery will often have a higher actual capacity than the comparable lead acid battery. This means that at the same capacity rating, the lithium will cost more, but a lower capacity lithium battery can be used for the same application at a lower price. The cost of ownership when considering the lifecycle further increases the value of the lithium battery when compared to a lead acid battery.[18]
Intellectual property
editThe root patents of LFP compounds are held by four organizations. University of Texas-Austin for the discovery of the material. Hydro-Québec, Université de Montréal and the French National Center for Scientific Research (CNRS) for the carbon coating that enhance its conductivity and actually makes LFP suitable for industrial developments.[19] These patents underlie mature mass production technologies. The largest production capacity is up to 250 tons per month. The key feature of Li
1−xMFePO
4 from A123 is the nano-LFP, which modifies its physical properties and adds noble metals in the anode, as well as the use of special graphite as the cathode.
The main feature of LiMPO
4 from Phostech is increased capacitance and conductivity by an appropriate carbon coating. The special feature of LiFePO
4 • zM from Aleees a high capacitance and low impedance obtained by the stable control of the ferrites and crystal growth. This improved control is realized by applying strong mechanical stirring forces to the precursors in high oversaturation states, which induces crystallization of the metal oxides and LFP.
In patent lawsuits in the US in 2005 and 2006, the University of Texas at Austin|University of Texas-Austin and Hydro-Québec claimed that LiFePO
4 as the cathode infringed their patents, US 5910382 and US 6514640. The patent claims involved a unique crystal structure and a chemical formula of the battery cathode material.
On April 7, 2006, A123 filed an action seeking a declaration of non-infringement and invalidity UT's patents. A123 separately filed two ex parte Reexamination Proceedings before the United States Patent and Trademark Office (USPTO), in which they sought to invalidate the patents based upon prior art.
In a parallel court proceeding, UT sued Valence Technology, Inc. ("Valence") - a company that commercializes LFP products that alleged infringement.
The USPTO issued a Reexamination Certificate for the '382 patent on April 15, 2008, and for the '640 patent on May 12, 2009, by which the claims of these patents were amended. This allowed the current patent infringement suits filed by Hydro-Quebec against Valence and A123 to proceed. After a Markman hearing, on April 27, 2011, the Western District Court of Texas held that the claims of the reexamined patents had a narrower scope than as originally granted. The key question was whether the earlier Goodenough's patents from the University of Texas (licensed to Hydro-Quebec) were infringed by A123, that had its own improved versions of LiFePO4 patents, that contained cobalt dopant. The end results was licensing of Googenough's patents by A123 under undisclosed terms.[20]
On December 9, 2008, the European Patent Office revoked Dr. Goodenough’s patent numbered 0904607. This decision basically reduced the patent risk of using LFP in European automobile applications. The decision is believed to be based on the lack of novelty.[21]
The first major large settlement was the lawsuit between NTT and the University of Texas-Austin (UT). In October 2008,[22] NTT announced that they would settle the case in the Japan Supreme Civil Court for $30 million. As part of the agreement, UT agreed that NTT did not steal the information and that NTT would share its LFP patents with UT. NTT’s patent is also for an olivine LFP, with the general chemical formula of A
yMPO
4 (A is for alkali metal and M for the combination of Co and Fe), now used by BYD Company. Although chemically the materials are nearly the same, from the viewpoint of patents, A
yMPO
4 of NTT is different from the materials covered by UT. A
yMPO
4 has higher capacity than LiMPO
4. At the heart of the case was that NTT engineer Okada Shigeto, who had worked in the UT labs developing the material, was accused of stealing UT’s intellectual property.
As of 2020, an organization named LifePO+C claims to own the key IP and offers licenses. It is a consortium between Johnson Matthey, the CNRS, University of Montreal, and Hydro Quebec.
Research
editPower density
editLFP has two shortcomings: low conductivity (high overpotential) and low lithium diffusion constant, both of which limit the charge/discharge rate. Adding conducting particles in delithiated FePO
4 raises its electron conductivity. For example, adding conducting particles with good diffusion capability like graphite and carbon[23] to LiMPO
4 powders significantly improves conductivity between particles, increases the efficiency of LiMPO
4 and raises its reversible capacity up to 95% of the theoretical values. However, addition of conductive additives also increases the "dead mass" present in the cell that does not contribute to energy storage. LiMPO
4 shows good cycling performance even under charge/discharge current as large as 5C.[24]
Stability
editCoating LFP with inorganic oxides can make LFP’s structure more stable and increase conductivity. Traditional LiCoO
2 with oxide coating shows improved cycling performance. This coating also inhibits dissolution of Co and slows the decay of LiCoO
2 capacity. Similarly, LiMPO
4 with an inorganic coating such as ZnO[25] and ZrO
2,[26] has a better cycling lifetime, larger capacity and better characteristics under rapid discharge. The addition of a conductive carbon increases efficiency. Mitsui Zosen and Aleees reported that addition of conducting metal particles such as copper and silver increased efficiency.[27] LiMPO
4 with 1 wt% of metal additives has a reversible capacity up to 140 mAh/g and better efficiency under high discharge current.
Metal substitution
editSubstituting other metals for the iron or lithium in LiMPO
4 can also raise efficiency. Substituting zinc for iron increases crystallinity of LiMPO
4 because zinc and iron have similar ionic radii.[28] Cyclic voltammetry confirms that LiFe
1−xM
xPO
4, after metal substitution, has higher reversibility of lithium ion insertion and extraction. During lithium extraction, Fe (II) is oxidized to Fe (III) and the lattice volume shrinks. The shrinking volume changes lithium’s returning paths.
Synthesis processes
editMass production with stability and high quality still faces many challenges.
Similar to lithium oxides, LiMPO
4 may be synthesized by a variety of methods, including: solid-phase synthesis, emulsion drying, sol-gel process, solution coprecipitation, vapor-phase deposition, electrochemical synthesis, electron beam irradiation, microwave process[vague], hydrothermal synthesis, ultrasonic pyrolysis and spray pyrolysis.
In the emulsion drying process, the emulsifier is first mixed with kerosene. Next, the solutions of lithium salts and iron salts are added to this mixture. This process produces nanocarbon particles.[29] Hydrothermal synthesis produces LiMPO
4 with good crystallinity. Conductive carbon is obtained by adding polyethylene glycol to the solution followed by thermal processing.[30] Vapor phase deposition produces a thin film LiMPO
4.[31] In flame spray pyrolysis FePO4 is mixed with lithium carbonate and glucose and charged with electrolytes. The mixture is then injected inside a flame and filtered to collect the synthesized LiFePO
4.[32]
Effects of temperature
editThe effects of temperature on lithium iron phosphate batteries can be divided into the effects of high temperature and low temperature.
Generally, LFP chemistry batteries are less susceptible to thermal runaway reactions like those that occur in lithium cobalt batteries; LFP batteries exhibit better performance at an elevated temperature. Research has shown that at room temperature (23 °C), the initial capacity loss approximates 40-50 mAh/g. However, at 40 °C and 60 °C, the capacity losses approximate 25 and 15 mAh/g respectively, but these capacity losses were spread over 20 cycles instead of a bulk loss like that in the case of room temperature capacity loss.[33]
However, this is only true for a short cycling timeframe. Later yearlong study has shown that despite LFP batteries having double the equivalent full cycle, the capacity fate rate increased with increasing temperature for LFP cells but the increasing temperature does not impact NCA cells or have a negligible impact on the aging of NMC cells.[34] This capacity fade is primarily due to the solid electrolyte interface (SEI) formation reaction being accelerated by increasing temperature.
LFP batteries are especially affected by decreasing temperature which possibly hamper their application in high-latitude areas. The initial discharge capacities for LFP/C samples at temperatures of 23, 0, -10, and -20 °C are 141.8, 92.7, 57.9 and 46.7 mAh/g with coulombic efficiency 91.2%, 74.5%, 63.6% and 61.3%. These losses are accounted for by the slow diffusion of lithium ions within electrodes and the formation of SEI that come with lower temperatures which subsequently increase the charge-transfer resistance on the electrolyte-electrode interfaces.[35] Another possible cause of the lowered capacity formation is lithium plating. As mentioned above, low temperature lowers the diffusion rate of lithium ions within the electrodes, allowing for the lithium plating rate to compete with that of intercalation rate. The colder condition leads to higher growth rates and shifts the initial point to lower state of charge which means that the plating process starts earlier.[36] Lithium plating uses up lithium which then compete with the intercalation of lithium into graphite, decreasing the capacity of the batteries. The aggregated lithium ions are deposited on the surface of electrodes in the form of “plates” or even dendrites which may penetrate the separators, short-circuiting the battery completely.[37]
See also
editReferences
edit- ^ Park, O. K.; Cho, Y.; Lee, S.; Yoo, H.-C.; Song, H.-K.; Cho, J., "Who Will Drive Electric Vehicles, Olivine or Spinel?", Energy Environ. Sci. 2011, volume 4, pages 1621-1633. doi:10.1039/c0ee00559b
- ^ Ozawa, Ryan (7 July 2015). "New Energy Storage Startup to Take Hawaii Homes Off-Grid". Hawaii Blog. Retrieved 2015-07-09.
- ^ "Google Looks to Batteries as Replacement for Diesel Generators". 16 December 2020.
- ^ Fedotov, Stanislav S.; Luchinin, Nikita D.; Aksyonov, Dmitry A.; Morozov, Anatoly V.; Ryazantsev, Sergey V.; Gaboardi, Mattia; Plaisier, Jasper R.; Stevenson, Keith J.; Abakumov, Artem M.; Antipov, Evgeny V. (2020-03-20). "Titanium-based potassium-ion battery positive electrode with extraordinarily high redox potential". Nature Communications. 11 (1): 1484. Bibcode:2020NatCo..11.1484F. doi:10.1038/s41467-020-15244-6. ISSN 2041-1723. PMC 7083823. PMID 32198379.
LiTiPO4F
- ^ Kim, Jongsoon (2012). "Thermal Stability of Fe-Mn Binary Olivine Cathodes for Li Rechargeable Batteries". Journal of Materials Chemistry. 22 (24). The Royal Society of Chemistry: 11964. doi:10.1039/C2JM30733B. Retrieved 19 Oct 2012.
- ^ Wang, J.; Sun, X., "Olivine Lifepo4: The Remaining Challenges for Future Energy Storage", Energy Environ. Sci. 2015, volume 8, pages 1110-1138. doi:10.1039/C4EE04016C
- ^ Masquelier, Christian; Croguennec, Laurence (2013). "Polyanionic (Phosphates, Silicates, Sulfates) Frameworks as Electrode Materials for Rechargeable Li (or Na) Batteries". Chemical Reviews. 113 (8): 6552–6591. doi:10.1021/cr3001862. PMID 23742145.
- ^ Manthiram, A.; Goodenough, J. B. (1989). "Lithium insertion into Fe2(SO4)3 frameworks". Journal of Power Sources. 26 (3–4): 403–408. Bibcode:1989JPS....26..403M. doi:10.1016/0378-7753(89)80153-3.
- ^ Manthiram, A.; Goodenough, J. B. (1987). "Lithium insertion into Fe2(MO4)3 frameworks: Comparison of M = W with M = Mo". Journal of Solid State Chemistry. 71 (2): 349–360. Bibcode:1987JSSCh..71..349M. doi:10.1016/0022-4596(87)90242-8.
- ^ "LiFePO
4: A Novel Cathode Material for Rechargeable Batteries", A.K. Padhi, K.S. Nanjundaswamy, J.B. Goodenough, Electrochemical Society Meeting Abstracts, 96-1, May, 1996, pp 73 - ^ “Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries” A. K. Padhi, K. S. Nanjundaswamy, and J. B. Goodenough, J. Electrochem. Soc., Volume 144, Issue 4, pp. 1188-1194 (April 1997)
- ^ Nature Materials, 2008, 7, 707-711.
- ^ Jugović, Dragana; Uskoković, Dragan (2009-05-15). "A review of recent developments in the synthesis procedures of lithium iron phosphate powders". Journal of Power Sources. 190 (2): 538–544. Bibcode:2009JPS...190..538J. doi:10.1016/j.jpowsour.2009.01.074. ISSN 0378-7753. Retrieved 2017-11-21.
- ^ a b Love, Corey T.; Korovina, Anna; Patridge, Christopher J.; Swider-Lyons; Karen E.; Twigg, Mark E.; Ramaker, David E. (2013). "Review of LiFePO
4 phase transition mechanisms and new observations from X-ray absorption spectroscopy". Journal of the Electrochemical Society. 160 (5): A3153–A3161. doi:10.1149/2.023305jes. - ^ Malik, R.; Abdellahi, A.; Ceder, G., "A Critical Review of the Li Insertion Mechanisms in LiFePO
4 Electrodes", J. Electrochem. Soc. 2013, volume 160, pages A3179-A3197. doi:10.1149/2.029305jes - ^ Rechargeable Li-ion batteries based on Olivine-structured (LiFePO4) cathode materials - Kumar et al., Nov 15, 2015, retrieved April 1, 2020
- ^ Warren, Chris (March 12, 2016). "What You Need To Know About LiFePO4 Batteries".
- ^ "Lead Acid Vs LiFePO4 Batteries". Power Sonic - Trusted Battery Solutions. 25 February 2020.
- ^ Basel, Clariant Ltd. "Especialidades químicas da Clariant". Clariant Ltd.
- ^ Claim Construction; Insights from the Lithium Battery Patent Infringement Case." Electrochemical Society Interface 29(2): 53-59; 10.1149.2/2.F07202if
- ^ "EPO Revokes Univ. Of Texas European Patent on Lithium Metal Phosphates; Boon for Valence".
- ^ "NTT Settles Lawsuit over Li-ion Battery Patents".
- ^ Deb, Aniruddha; Bergmann, Uwe; Cairns, Elton J.; Cramer, Stephen P. (June 2004). "Structural Investigations of LiFePO 4 Electrodes by Fe X-ray Absorption Spectroscopy". The Journal of Physical Chemistry B. 108 (22): 7046–7051. doi:10.1021/jp036361t.
- ^ Haas, O.; Deb, A.; Cairns, E. J.; Wokaun, A. (2005). "Synchrotron X-Ray Absorption Study of LiFePO[sub 4] Electrodes". Journal of the Electrochemical Society. 152 (1): A191. doi:10.1149/1.1833316.
- ^ Kwon, Sang Jun; Kim, Cheol Woo; Jeong, Woon Tae; Lee, Kyung Sub (October 2004). "Synthesis and electrochemical properties of olivine LiFePO4 as a cathode material prepared by mechanical alloying". Journal of Power Sources. 137 (1): 93–99. Bibcode:2004JPS...137...93K. doi:10.1016/j.jpowsour.2004.05.048.
- ^ Dominko, R.; Bele, M.; Gaberscek, M.; Remskar, M.; Hanzel, D.; Goupil, J.M.; Pejovnik, S.; Jamnik, J. (February 2006). "Porous olivine composites synthesized by sol–gel technique". Journal of Power Sources. 153 (2): 274–280. Bibcode:2006JPS...153..274D. doi:10.1016/j.jpowsour.2005.05.033.
- ^ León, B.; Vicente, C. Pérez; Tirado, J. L.; Biensan, Ph.; Tessier, C. (2008). "Optimized Chemical Stability and Electrochemical Performance of LiFePO[sub 4] Composite Materials Obtained by ZnO Coating". Journal of the Electrochemical Society. 155 (3): A211–A216. doi:10.1149/1.2828039.
- ^ Liu, H.; Wang, G.X.; Wexler, D.; Wang, J.Z.; Liu, H.K. (January 2008). "Electrochemical performance of LiFePO4 cathode material coated with ZrO2 nanolayer". Electrochemistry Communications. 10 (1): 165–169. doi:10.1016/j.elecom.2007.11.016.
- ^ Croce, F.; D' Epifanio, A.; Hassoun, J.; Deptula, A.; Olczac, T.; Scrosati, B. (2002). "A Novel Concept for the Synthesis of an Improved LiFePO[sub 4] Lithium Battery Cathode". Electrochemical and Solid-State Letters. 5 (3): A47–A50. doi:10.1149/1.1449302.
- ^ Ni, J.F.; Zhou, H.H.; Chen, J.T.; Zhang, X.X. (August 2005). "LiFePO4 doped with ions prepared by co-precipitation method". Materials Letters. 59 (18): 2361–2365. doi:10.1016/j.matlet.2005.02.080.
- ^ Cho, Tae-Hyung; Chung, Hoon-Taek (June 2004). "Synthesis of olivine-type LiFePO4 by emulsion-drying method". Journal of Power Sources. 133 (2): 272–276. Bibcode:2004JPS...133..272C. doi:10.1016/j.jpowsour.2004.02.015.
- ^ Hamid, N.A.; Wennig, S.; Hardt, S.; Heinzel, A.; Schulz, C.; Wiggers, H. (October 2012). "High-capacity cathodes for lithium-ion batteries from nanostructured LiFePO4 synthesized by highly-flexible and scalable flame spray pyrolysis". Journal of Power Sources. 216: 76–83. Bibcode:2012JPS...216...76H. doi:10.1016/j.jpowsour.2012.05.047.
- ^ Andersson, Anna S; Thomas, John O; Kalska, Beata; Häggström, Lennart (2000). "Thermal Stability of LiFePO4 -Based Cathodes". Electrochemical and Solid-State Letters. 3: 66–68. doi:10.1149/1.1390960. Retrieved 2021-11-18.
- ^ Preger, Yulia; Barkholtz, Heather M.; Fresquez, Armado; Campbell, Danel L.; Juba, Benjamin W. (2020). "Degradation of Commercial Lithium-Ion Cells as a Function of Chemistry and Cycling Conditions". Journal of the Electrochemical Society. 167 (12): 120532. Bibcode:2020JElS..167l0532P. doi:10.1149/1945-7111/abae37. S2CID 225506214.
- ^ Rui, X.H.; Jin, Y.; Feng, X.Y.; Zhang, L.C.; Chen, C.H. (February 2011). "A comparative study on the low-temperature performance of LiFePO4/C and Li3V2(PO4)3/C cathodes for lithium-ion batteries". Journal of Power Sources. 196 (4): 2109–2114. doi:10.1016/j.jpowsour.2010.10.063. ISSN 0378-7753. Retrieved 2021-11-18.
- ^ Petzl, Mathias; Danzer, Michael A. (May 2014). "Nondestructive detection, characterization, and quantification of lithium plating in commercial lithium-ion batteries". Journal of Power Sources. 254: 80–87. Bibcode:2014JPS...254...80P. doi:10.1016/j.jpowsour.2013.12.060. ISSN 0378-7753. Retrieved 2021-11-18.
- ^ Liu, Huaqiang; Wei, Zhongbao; He, Weidong; Zhao, Jiyun (October 2017). "Thermal issues about Li-ion batteries and recent progress in battery thermal management systems: A review". Energy Conversion and Management. 150: 304–330. doi:10.1016/j.enconman.2017.08.016. ISSN 0196-8904. Retrieved 2021-11-18.
Category:Lithium compounds
Category:Iron(II) compounds
Category:Phosphates
Category:Rechargeable batteries